Give the IUPAC name for each of the following molecules. Which of these compounds could be named using ortho, meta, or para as a prefix? (a) (b) (c) (d) NO2 (e) (f) (g) Br CI O,N- CI
Give the IUPAC name for each of the following molecules. Which of these compounds could be named using ortho, meta, or para as a prefix? (a) (b) (c) (d) NO2 (e) (f) (g) Br CI O,N- CI
Chapter12: Unsaturated Hydrocarbons
Section: Chapter Questions
Problem 12.56E
Related questions
Question
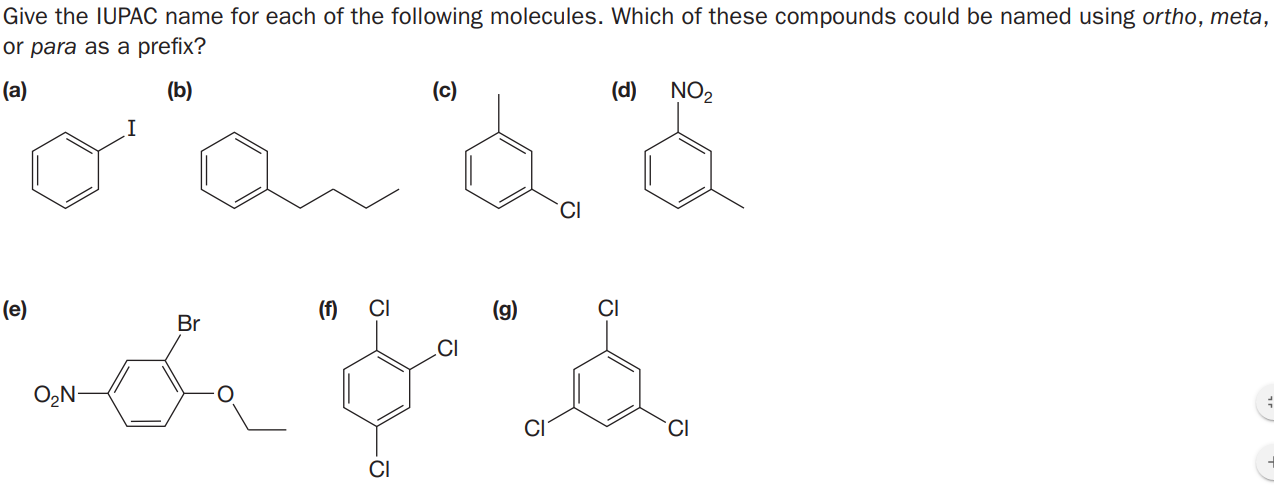
Transcribed Image Text:Give the IUPAC name for each of the following molecules. Which of these compounds could be named using ortho, meta,
or para as a prefix?
(a)
(b)
(c)
(d)
NO2
(e)
(f)
(g)
Br
CI
O,N-
CI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

