Give the product of the bimolecular elimination from each of the following isomeric halogenated compounds. А н Br KO BU Снз HOLBU +Br В н CH3 One of these compounds undergoes elimination 50x faster than the other. Which one and why? I.
Give the product of the bimolecular elimination from each of the following isomeric halogenated compounds. А н Br KO BU Снз HOLBU +Br В н CH3 One of these compounds undergoes elimination 50x faster than the other. Which one and why? I.
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 27VC
Related questions
Question
In this question, will A undergo a fast elimination product since it is on a dashed bond?
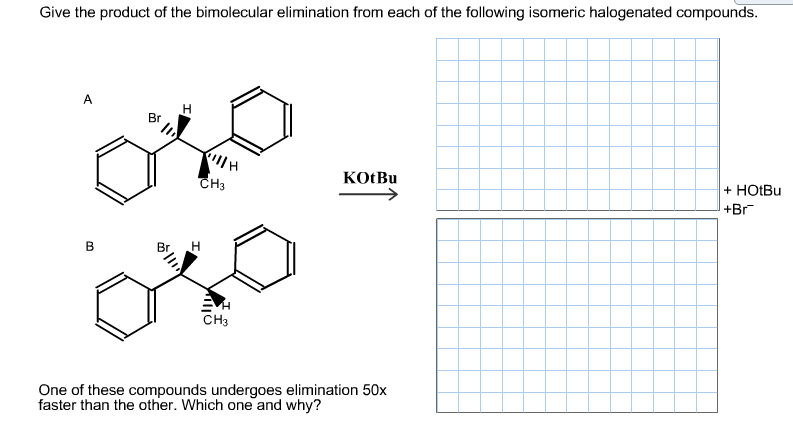
Transcribed Image Text:Give the product of the bimolecular elimination from each of the following isomeric halogenated compounds.
А
н
Br
KO BU
Снз
HOLBU
+Br
В
н
CH3
One of these compounds undergoes elimination 50x
faster than the other. Which one and why?
I.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

