given: (i) (iii) (iv) Which one is more stable? Briefly explain your answer. Use a suitable formula to estimate the amount of conformers (in percent) in which the methyl group is in axial and equatorial position at 298 K. Estimate also the energy that would be needed to have a ratio of 99.9% to 0.1%. What is the meaning of the calculated percentage of each particular conformer?
given: (i) (iii) (iv) Which one is more stable? Briefly explain your answer. Use a suitable formula to estimate the amount of conformers (in percent) in which the methyl group is in axial and equatorial position at 298 K. Estimate also the energy that would be needed to have a ratio of 99.9% to 0.1%. What is the meaning of the calculated percentage of each particular conformer?
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter16: Aldehydes And Ketones
Section: Chapter Questions
Problem 16.71P
Related questions
Question
Don’t answer (I)
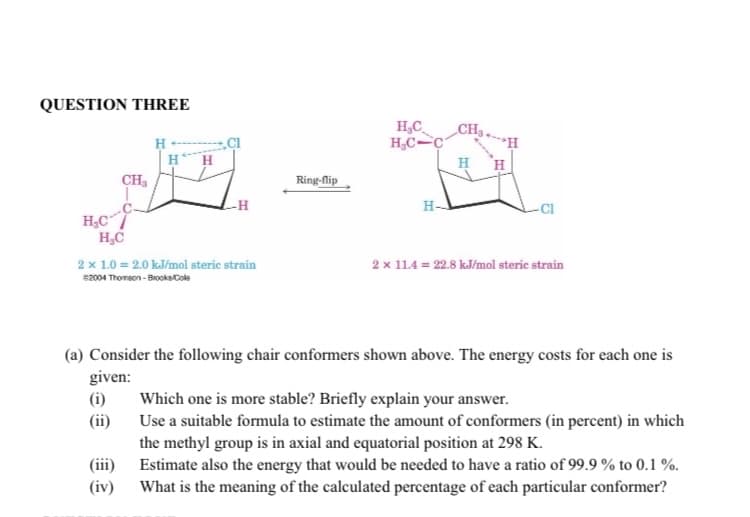
Transcribed Image Text:QUESTION THREE
H₂CC
H₂C
CH₂
(i)
(ii)
H
2 x 1.0 = 2.0 kJ/mol steric strain
52004 Thomson-Brooks Cole
(iii)
(iv)
-H
Ring-flip
H₂C
H₂C-C
H-
CH₂
-CI
(a) Consider the following chair conformers shown above. The energy costs for each one is
given:
2 x 11.4 = 22.8 kJ/mol steric strain
Which one is more stable? Briefly explain your answer.
Use a suitable formula to estimate the amount of conformers (in percent) in which
the methyl group is in axial and equatorial position at 298 K.
Estimate also the energy that would be needed to have a ratio of 99.9% to 0.1%.
What is the meaning of the calculated percentage of each particular conformer?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
