Given the balanced equation: HBr(aq) + NaOH(aq) NaBr(aq) + H2O) and remembering that Molarity = moles/liter OR mmoles/mL (1) (a) Calculate the number of mmoles of HBr in 100.0 mL of 0.250 MHBR (b) Calculate the number of mmoles of NaOH in 100.0 mL of 0.250 MNAOH © When these two solutions are mixed the acid and base should neutralize one another exactly. This means that all of the acid and base are completely used up; either one could be considered a "limiting reactant". Starting with the mmoles of either the acid or base, calculate the number of mmoles of salt produced by the reaction.
Given the balanced equation: HBr(aq) + NaOH(aq) NaBr(aq) + H2O) and remembering that Molarity = moles/liter OR mmoles/mL (1) (a) Calculate the number of mmoles of HBr in 100.0 mL of 0.250 MHBR (b) Calculate the number of mmoles of NaOH in 100.0 mL of 0.250 MNAOH © When these two solutions are mixed the acid and base should neutralize one another exactly. This means that all of the acid and base are completely used up; either one could be considered a "limiting reactant". Starting with the mmoles of either the acid or base, calculate the number of mmoles of salt produced by the reaction.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section: Chapter Questions
Problem 120SCQ
Related questions
Question

Transcribed Image Text:Given the balanced equation:
HBr(aq) + NaOH(aq)
NaBr(ag) + H2O()
and remembering that
Molarity = moles/liter
OR
mmoles/mL
(1) (a) Calculate the number of mmoles of HBr in 100.0 mL of 0.250 MHB.
(b) Calculate the number of mmoles of NaOH in 100.0 mL of 0.250 MNAOH
©) When these two solutions are mixed the acid and base should neutralize one
another exactly. This means that all of the acid and base are completely used up;
ing reactant". Starting with the mmoles of
either the acid or base, calculate the number of mmoles of salt produced by the
either one could be considered a "li
reaction.
(d) Using the mmoles of salt produced and the total volume in mL of solution (from the
mixing of the acid and base solutions), calculate the molarity of the salt solution
produced by this reaction.
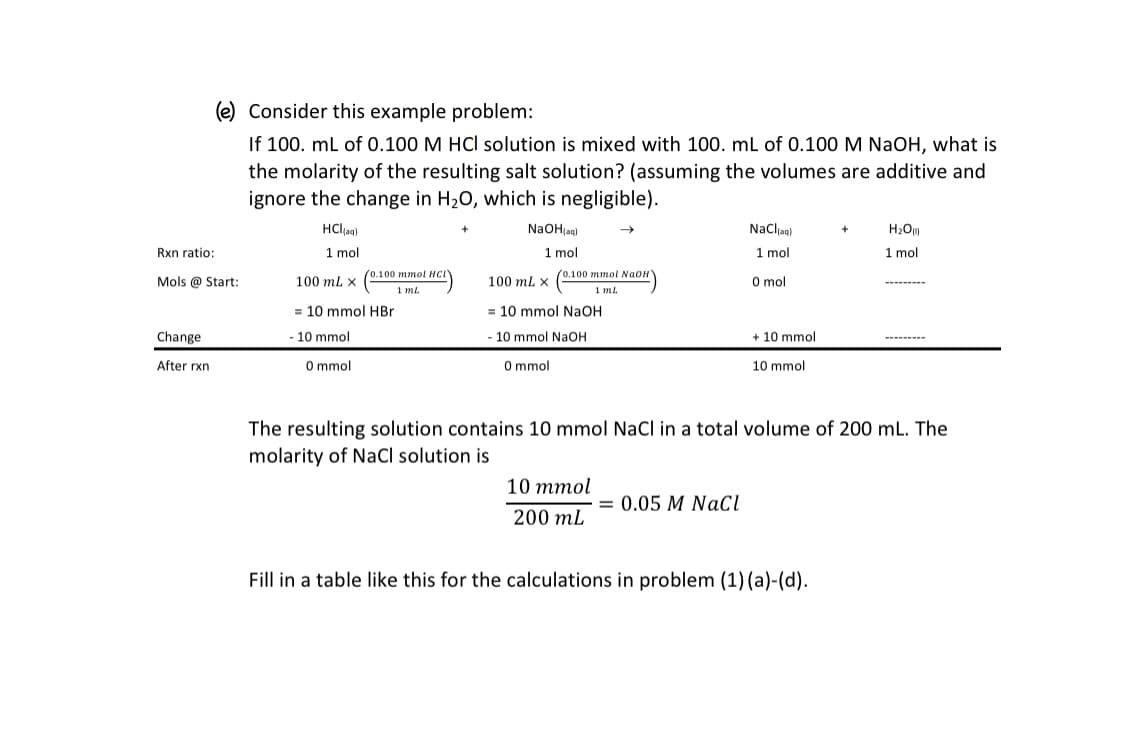
Transcribed Image Text:(e) Consider this example problem:
If 100. ml of 0.100 M HCl solution is mixed with 100. mL of 0.100 M NaOH, what is
the molarity of the resulting salt solution? (assuming the volumes are additive and
ignore the change in H20, which is negligible).
HClag)
NaOH;ag)
Nacla)
H2Ou
Rxn ratio:
1 mol
1 mol
1 mol
1 mol
100 ml. x (0.100 mmol Naon
1 ml
(0.100 ттol Hнс
Mols @ Start:
100 ml x
O mol
1 ml
= 10 mmol HBr
= 10 mmol NaOH
Change
- 10 mmol
10 mmol NaOH
10 mmol
After rxn
O mmol
O mmol
10 mmol
The resulting solution contains 10 mmol NaCl in a total volume of 200 mL. The
molarity of NaCl solution is
10 mmol
= 0.05 M NaCl
200 mL
Fill in a table like this for the calculations in problem (1) (a)-(d).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning