Given the table below, answer the following questions: Group Number (Old) Group Nu Atomic Element Electron Configuration Ovana 1 Number Не 2 1s? 8A 18 Ne 10 1s 2s 2p 8A 18 2 Ar 18 1s 2s 2p 3s? 3p 8A 18 3 Kr 36 1s 2s2 2p 3s? 3p 3d10 4s2 4p 8A 18 4 1s 2s? 2p 3s? 3p 3d10 4s24p 4d10 4f145s 5p 1s 2s? 2p 3s? 3p 3d10 4s2 4ps 4d10 4f145s2 5p6 5d10 6s2 6p Хе 54 8A 18 5 Ra 86 8A 18 6 1. To what group in the periodic table do these elements belong? 2.These elements are called the noble gases. Why? What is the common characteristic of these gases? 3. Light bulbs are filled with Argon gas rather than oxygen gas. Why? 4. From the electron configuration, how many valence electrons do noble gases have? 5. Can we relate the number of valence electrons with the stability of the element?
Given the table below, answer the following questions: Group Number (Old) Group Nu Atomic Element Electron Configuration Ovana 1 Number Не 2 1s? 8A 18 Ne 10 1s 2s 2p 8A 18 2 Ar 18 1s 2s 2p 3s? 3p 8A 18 3 Kr 36 1s 2s2 2p 3s? 3p 3d10 4s2 4p 8A 18 4 1s 2s? 2p 3s? 3p 3d10 4s24p 4d10 4f145s 5p 1s 2s? 2p 3s? 3p 3d10 4s2 4ps 4d10 4f145s2 5p6 5d10 6s2 6p Хе 54 8A 18 5 Ra 86 8A 18 6 1. To what group in the periodic table do these elements belong? 2.These elements are called the noble gases. Why? What is the common characteristic of these gases? 3. Light bulbs are filled with Argon gas rather than oxygen gas. Why? 4. From the electron configuration, how many valence electrons do noble gases have? 5. Can we relate the number of valence electrons with the stability of the element?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 34PS: Identify the element that corresponds to each of the simplified photoelectron spectral data given...
Related questions
Question
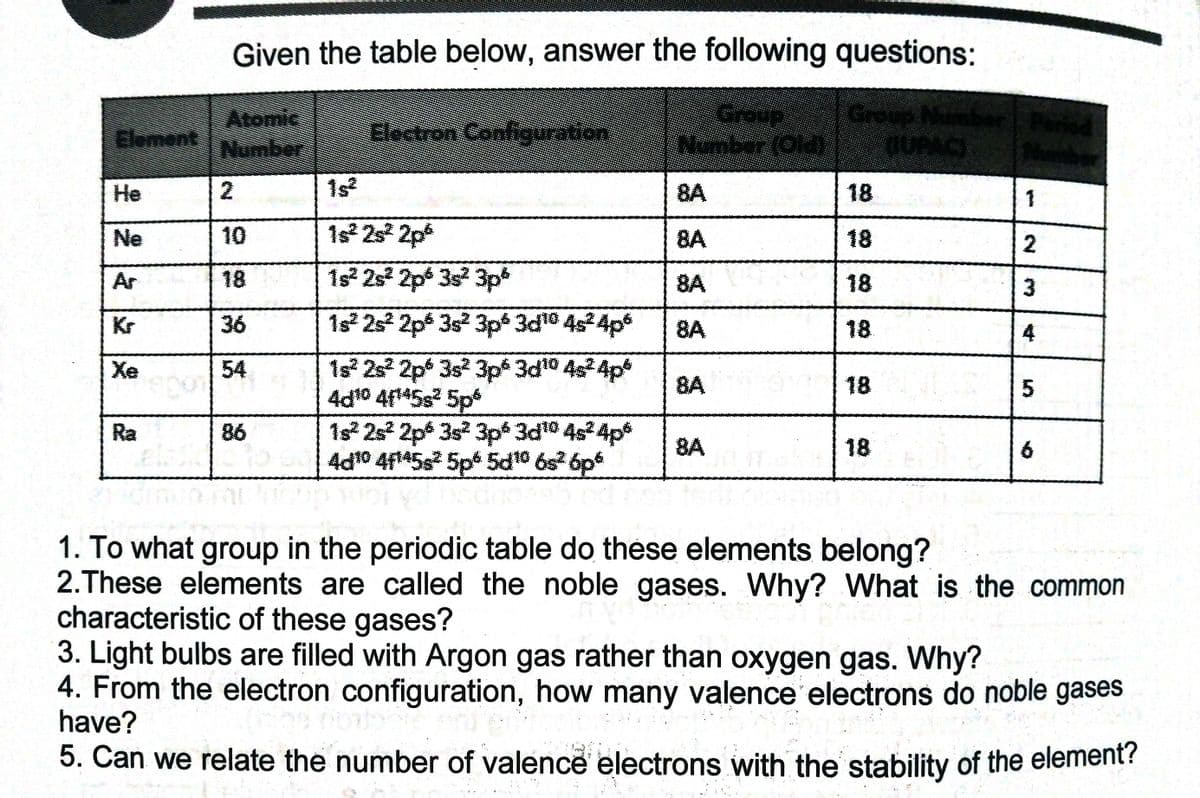
Transcribed Image Text:Given the table below, answer the following questions:
Atomic
Number
Group
Number (Old)
Group N
OUPAC
Element
Electron Configuration
He
2.
1s
8A
18
Ne
10
1s 2 2p*
BA
18
Ar
18
1s 2s 2p* 3s 3p*
8A
18
3
Kr
36
1s 2s 2p 3s 3p 3d@ 4s24p*
8A
18.
4
18 2s 2p* 3s 3p* 3d" 4s4p*
4d0 445s 5p*
1s 2s 2p 3s? 3p* 3d 4s 4p%
4d10 4f145s Sp% 5d1 6s? 6p
Xe
54
8A
18
Ra
86
8A
18
1. To what group in the periodic table do these elements belong?
2.These elements are called the noble gases. Why? What is the common
characteristic of these gases?
3. Light bulbs are filled with Argon gas rather than oxygen gas. Why?
4. From the electron configuration, how many valence electrons do noble gases
have?
5. Can we relate the number of valence electrons with the stability of the element?
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning