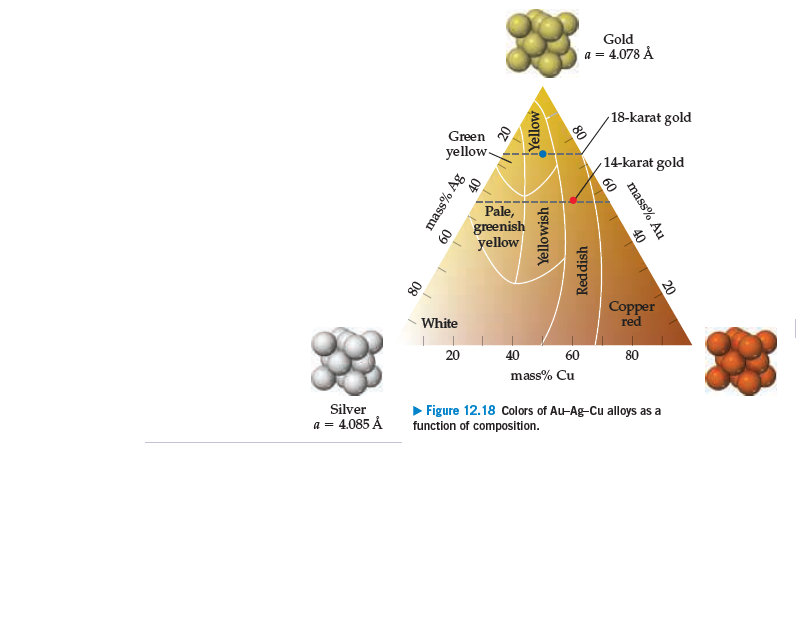
Gold a = 4.078 Å 18-karat gold Green yellow- 14-karat gold 60 Pale, greenish yellow Copper red White 20 40 60 80 mass% Cu Silver > Figure 12.18 Colors of Au-Ag-Cu alloys as a a = 4.085 Å function of composition. mass% Au 80 40 20 Yellow Reddish OF 40 Yellowish 09 mass% Ag
Gold a = 4.078 Å 18-karat gold Green yellow- 14-karat gold 60 Pale, greenish yellow Copper red White 20 40 60 80 mass% Cu Silver > Figure 12.18 Colors of Au-Ag-Cu alloys as a a = 4.085 Å function of composition. mass% Au 80 40 20 Yellow Reddish OF 40 Yellowish 09 mass% Ag
Chapter4: Calculations Used In Analytical Chemistry
Section: Chapter Questions
Problem 4.32QAP
Related questions
Question
The karat scale used to describe gold alloys is based on
mass percentages. (a) If an alloy is formed that is 50 mol%
silver and 50 mol% gold, what is the karat number of the
alloy? Use Figure 12.18 to estimate the color of this alloy.
(b) If an alloy is formed that is 50 mol% copper and 50
mol% gold, what is the karat number of the alloy? What is
the color of this alloy?
Transcribed Image Text:Gold
a = 4.078 Å
18-karat gold
Green
yellow-
14-karat gold
60
Pale,
greenish
yellow
Copper
red
White
20
40
60
80
mass% Cu
Silver
> Figure 12.18 Colors of Au-Ag-Cu alloys as a
a = 4.085 Å
function of composition.
mass% Au
80
40
20
Yellow
Reddish
OF
40
Yellowish
09
mass% Ag
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

