Q: A 17.3 mg sample of an organic compound (a non-electrolyte) was ground up with 420 mg of camphor to…
A: First to calculate Molality Then using the formula for depression in freezing point to calculate the…
Q: Which of the following shows the increasing boiling point oh halogens? A. F2 < I2 < Br2 < Cl2 B. I2…
A: Intermoleular forces can be defined as the forces that exist between two or more than two molecules.…
Q: normality
A:
Q: Give the clear handwritten answer .
A: Structure of Z-isomer can be drawn in the following way
Q: limiting reactant
A:
Q: Solutions of magnesium sulfate and potassium phosphate react to form a precipitate. What volume, in…
A: Given:: Molarity of MgSO4 = 3.74 mol L-1 Moles of ppt = 63.7 mol Volume of MgSO4= ?
Q: 1. The structures of six organic compounds are shown below. H EH H2N (b) (c) NH2 NH2 H2N OH (d) (e)…
A: A molecular formula made of the chemical symbols of the constituent elements of a certain compound…
Q: Complete the following nuclear bombardment equation by filling in the nuclear symbol for the missing…
A:
Q: t a certain temperature the vapor pressure of pure acetyl bromide (CH,COB1) is measured to be 172.…
A: Given- Pressure= 172 mmHg Weight of acetyl bromide =121 gram Weight of thiophene= 86.9 g
Q: the ionization constant for HCN is 4.5 x 10^-10. what is the pH of a 0.43 molar solution of sodium…
A: PH = -log [H+]
Q: Time (min) Temperature (°C) 25.00 1 22.10 2. 21.40 3. 20.00 4 19.50 17.40 6. 15.60 12.40 8. 10.70…
A: Answer: This question is based on the basic understanding of the process of phase chnage.
Q: What is the molar concentration of a 15% glycerol solution? Chemical formula glycerol: C3H8O3 MWs:…
A:
Q: Supply the correct word/s that complete/s the sentence: “In distilling a mixture of two miscible…
A: Distillation is the process by which liquid mixtures are separated based on the difference in their…
Q: A. Do gioUI 17. The unit of boiling point elevation constant (Kb ) is A. K/ kg*mol B. K/ kg+mol C.…
A: We would use formula of elevation of boiling point to determine unit of Kb .
Q: Write a bond-line formula for each of the following compounds. 1. Cyclopentylcyclopentane 2.…
A: Detail solution is given below
Q: Before you proceed with the titration proper, you must first determine the exact concentration of…
A: 3. Answer - The correct option is - (C) - standardization Explanation - According to the question -…
Q: Complete the ICE table in concentrations (or pressure, if requested) for the following reaction if…
A:
Q: Chromium is a metal which exhibits a body-centered cubic crystalline structure. A. Use the cube…
A: Given,
Q: For each of the following compounds, decide whether the compound's solubility in aqueous solution…
A: Here we have to predict the solubility of insoluble salt in acidic and basic medium. If anion of…
Q: The letter labels closest to the points marked with a dot refer specifically to those points. The…
A:
Q: Pentaborane, Bs Ha, was once i Studied asYatpotentials roCket fuel.)calculate Srutthe heat given…
A:
Q: If you have 1 mol Xe and 1 mol F2; how many moles of XeF2 can you create in the following chemical…
A:
Q: The thiourea in a 1.563 g sample of an organic material was extracted into a dilute H2SO4 solution…
A: Given, Mass of thiourea = 1.563 g Molarity of Hg2+ = 0.009284 M Volume of Hg2+ = 36.43 mL Given…
Q: Suggest the balanced chemical equations for the reaction between the following pairs of compounds.…
A:
Q: Write a bond-line formula for each of the following compounds. 1. Cyclopentylcyclopentane 2.…
A: Bond-line formula are given below
Q: 3.Which is the conjugate acid in the equation? N2H4 + H2O E N2H5 + OH- А. N2H5 В. N2H4 С. OH D. H20
A: Given, Options are :
Q: Which of the following statements is incorrect about this reaction: CH3-CH=CH2 + HBr -->…
A:
Q: Problem 1 (Show your work for all parts of answer.) A student did an analysis for clomazone in soil…
A: Answer: Given data- Flow of nitrogen to exactly 10 ml and 50 ml of ethyl acetate.
Q: 2. Consider the five esters below with formula C 6H 12 O 2: (a) (d) (e) (i) Whic h is/are made from…
A:
Q: Given the FTIR Spectra picture, match the following bands to its corresponding interpretation.…
A: FT-IR streching depends on the strength and polarity of the bonds, More strong and polar is the bond…
Q: Now consider positron emission. "E +,8+R In which direction would a nucleus move on this chart if it…
A: When a nucleus is unstable and emits radiations, it is called radioactivity. It depends on n/p…
Q: СНЗОН ? NaOCH3 Isatoic Anhydride O:
A:
Q: H3C N. Он H3C°
A: Given,
Q: Chemistry A 20.55 mL sample of .63 M HCL is titrated with .63 M NaOH. Calculate the pH after the…
A: Neutralisation reaction is reaction where 1 mole of acid react with 1 mole of base to form salt and…
Q: Give IUPAC names for the following alkyl halides:
A:
Q: A 50.00-mL aliquot of 0.1000 M NaOH is titrated with 0.1000 M HCl. Calculate the pH of the solution…
A:
Q: Name the aldehyde displayed below using the IUPAC system. Cl O O 3-chlorohexanone 4-chloro-6-hexanal…
A: IUPAC nomenclature is done by looking upon the most prior group and position of branching
Q: lease use excel to calculate Fe analysis by ICPMS. 1') Generate a standard curve 2') calculate the…
A:
Q: If a student is titrating 10.0 mL of 1 M HNO3 with 1M NaOH and another students is titrating 10.0…
A:
Q: 1. A 1.000 g sample containing Na,C,O, (MM=134 mg/mmol) is titrated with 40.00 mL of 0.0200 M KMNO,…
A: It is an application of redox titration where a redox reaction occur between KMnO4 as titrant and…
Q: Given the equation: Na3PO4 + 3 KOH --> 3 NaOH + K3PO4 What is the first step in determining how much…
A: Using the sticheometric coefficient to calculate all.
Q: Consider the proposed mechanism shown below for the reaction between nitrogen dioxide and carbon…
A: Rate equation (or) rate law: The equation which gives exact mathematical relationship between rat…
Q: Gaseous butane (CH,(CH,) CH,) reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide…
A:
Q: For each system listed in the first column of the table below, decide (if possible) whether the…
A: Given:
Q: TQhB66q?1oB O CHEMICAL REACTIONS Percent yield of chemical reactions OOOC 15 quid octane…
A: Given : We have to calculate the percentage yield.
Q: Br2 light or heat
A: Given reaction is : Complete the reaction and gives the major product of the reaction = ?
Q: The molar solubility of calcium chromate in a 0.163 M calcium acetate solution is М.
A:
Q: 4. A 0.5501-g sample of primary-standard-grade Na2CO3 required 35.87 mL of H2SO4 solution to reach…
A: Given, Mass of Na2CO3 = 0.5501 g Volume of H2SO4 used = 35.87 mL Reaction is : CO32- + 2H+…
Q: An atom gained an electron, thus, becoming negatively charged. If it was then attracted to a polar…
A: The attraction forces between the different molecules are known as intermolecular forces.
Q: The volume of a gas sample is 866 ml at 11 atm and 467 K. What volume, in ml, will it occupy at 2…
A: According to Boyle's law, At constant temperature, the volume of a gas varies inversely with the…
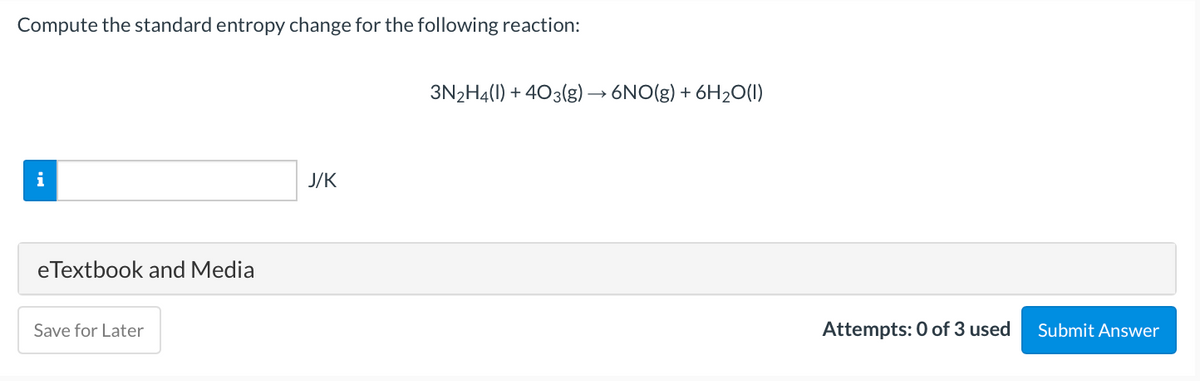
Step by step
Solved in 2 steps with 2 images

- calculate the standard entropy change for the following reqction at 25*C. round to 4 significant figures. 2S(s,rhombic)+ 3O2(g) --> 2SO3(g)Use Entropy values from the Thermodynamic Data Table in Resource Section of Atkin's Physical Chemistry to calculate ΔS° (in J/K) for the combustion of propane.C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) Answer in 4 significant figures.Solid iodine, I2(s), has an enthalpy of sublimation ΔHsubl = 62.4 kJ mol-1. Suppose 5.36 g of iodine at a constant temperature of 35.0 oC is allowed to sublime into the atmosphere at a temperature of 50.8 oC. 1.Calculate the entropy change of the system (i.e. the iodine) in J K-1. Enter your answer into the first answer field in accordance with the question statement
- Consider the reaction:2C2H6(g) + 7O2(g)4CO2(g) ---------> 6H2O(g)Using standard absolute entropies at 298K, calculate the entropy change for the system when 1.69 moles of C2H6(g) react at standard conditions. S°system = _______ J/KAssume that solutions of ethylbenzene : benzene behave ideally. a) Calculate the entropy of mixing if 40 g of ethylbenzene is mixed into 50 g of benzene.b) At room temperature (298 K), what is ΔmixG for mixing 40 g of ethylbenzene (PhEt) and 50 g of benzene (PhH)?c) Would you notice a temperature change associated with the process in parts a) & b)?d) Instead you mix 40 g of benzyl alcohol (PhMeOH) into 50 g of benzene (PhH). Let’s denote the difference between this process and the process in part b) as:ΔΔG = ΔmixG[ PhMeOH ∶ PhH ] − ΔmixG[ PhEt ∶ PhH ]What do you expect the sign of ΔΔG to be? ( ΔΔG < 0, ΔΔG ≈ 0, or ΔΔG > 0 )Briefly justify your answer.Calculate the standard reacton entropy at 298K of the following: 1. CH3CHO (g) + O2 -----→ CH3COOH (l) 2. Sucrose [C12H22O11(s)] + O2(g) ----→ CO2 (g) + H2O (l) 3. Zn(s) + Cu2+ (aq) ---→ Zn2+ (aq) + Cu (s)
- Assume that solutions of ethylbenzene : benzene behave ideally. a) Calculate the entropy of mixing if 40 g of ethylbenzene is mixed into 50g of benzene.b) At room temperature (298 K), what is ΔmixG for mixing 40g of ethylbenzene (PhEt) and 50g of benzene (PhH)?c) Would you notice a temperature change associated with the process in parts a) & b)?d) Instead, you mix 40g of benzyl alcohol (PhMeOH) into 50g of benzene (PhH). Let’s denote the difference between this process and the process in part b) as: ΔΔG = ΔmixG [PhMeOH ∶ PhH] − ΔmixG [PhEt ∶ PhH] What do you expect the sign of ΔΔG to be? (ΔΔG<0, ΔΔG≈0, or ΔΔG>0)Briefly justify your answer.Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction entropy of the following chemical reaction: →+Al2O3s3H2g+2Als3H2Og Round your answer to zero decimal places. H2 0 , 0, 130When nitric acid is produced industrially, nitrogen monoxide, NO, is first formed at high temperature. Bakefetr reacts NO on cooling further with oxygen to nitrogen dioxide: 2 NO(g) + O2 ⇌ 2 NO2 (g) Table 1: Thermodynamic data at 25°C. Bond ΔfHom Som Cop,m NO(g) 90.25 210.76 29.34 O2(g) 0.00 205.14 29.36 NO2(g) 33.18 240.06 37.20 1) Calculate (with all relevant intermediate calculations) the standard reaction Gibbs free energy, ΔrG25o, for reaction (1) at 25°C from the data in Table 1 2) Calculate (with all relevant intermediate calculations) the equilibrium constant K25, for reaction (1) at 25°C. 3) Industrially, however, the reaction does not proceed at 25°C but at 500°C. Therefore, calculate (with all relevant intermediate calculations) the standard reaction Gibbs free energy, ΔrG500o, for reaction (1) at 500°C under the assumption that the standard molar heat capacities, Cop, in Table 1 are independent of temperature in the interval [25°C, 500°C]
- Benzene (C6H6) has a melting point of 5.50C and an enthalpy of fusion of 10.04kJmol-1 at 25.00C. The molar heat capacities at constant pressure for solid and liquid benzene are 100.4JK-1mol-1 and 133.0JK-1mol-1, respectively. Calculate the change of entropy of system and change of entropy of the surrounding at 100C for the reaction of C6H6(l)---->C6H6(s)Consider the reaction:1. 2SO2(g) + O2(g)2SO3(g)Using standard absolute entropies at 298K, calculate the entropy change for the system when 2.06 moles of SO2(g) react at standard conditions. S°system = J/K Submit Answer 2. Consider the reactionFe2O3(s) + 3H2(g)2Fe(s) + 3H2O(g)Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 1.80 moles of Fe2O3(s) react at standard conditions. S°surroundings = J/K Submit AnswerA 1.0 mol of Cu metal at T = 90°C is dropped into a calorimeter containing 3.5 moles of H2O at T = 25°C. The calorimeter is sealed to the outside environment with a negligible heat capacity. The heat capacity of liquid water is 75.0 J/Kmol and for Cu is 25.0 J/Kmol. a) What is the entropy change of the Cu? b) What is the entropy change of the water?

