Q: How many moles of KCIO, solid must be reacted according to the following balanced chemical reaction…
A: Potassium chlorate decomposes into potassium chloride and oxygen gas. The equation for the balanced…
Q: C. How much heat is produced when 85 g of nitrogen reacts with excess hydrogen according to the…
A: Ans Balanced chemical equation N2 + 3H2 ------------------------> 2NH3 Given that H2 in excess to…
Q: Please answer all the guide questions. Thank you! Guide questions: 1. Is the reaction exothermic or…
A: 1.)The reactions that release energy into the surroundings are known as exothermic reactions while…
Q: In the following reaction, how much heat is generated when 3.21 moles of CH4 are burned? CH4 (g) + 2…
A: CH4g+2O2g→ CO2g+2H2Og △ H = 802KJmolAs 1 mole of CH4 heat released =…
Q: In the following reaction, how much heat is generated when 6.57 moles of CH₄ are burned? CH₄ (g) + 2…
A: The change in enthalpy of a reaction measures the amount of heat absorbed or released during the…
Q: A 7.03 g sample of an unknown salt (MM = 116.82 g/mol) is dissolved in 150.00 g water in a coffee…
A: We know during the process of any reaction, heat gained = heat lost . For above the formula used…
Q: How much heat is involved when 68.0 g of N2 are reacted in the reaction: 2N2(g) + 5O2(g) + 2H2O(l) →…
A: Given, the chemical reaction,2N2(g)+5O2(g)+2H2O(l)→4HNO3(aq) ; ∆H =-256 kJamount of heat evolved…
Q: Formation of carbon dioxide gas from (graphite) and oxygen, yields 393. 5 kJ of heat.
A: To find: The equation for the formation of carbon dioxide gas from (graphite) and oxygen yields…
Q: solve the attached
A: First, calculate the number of moles of N2: No of moles = Mass/Molar mass = 68.0g/(28.0gmol-1) =…
Q: How much heat is needed to heat 1.0 mole (32.00 g) of oxygen from -250 C to -200 c?
A: Given information: Mass of oxygen = 32 g Initial temperature = -250∘C Final temperature = -200∘C
Q: calculate the heat of reaction AH for the following reaction:
A: CH4 (g)+ 2 O2 (g)------->CO2 (g)+ 2 H20(l) required energy to break bonds in molecule(bond…
Q: How much heat in kilojoules is released when 209.0 g hydrogen bromide (HBr) is dissolved if its…
A: Given: Molar heat of solution = -85.1 kJ/mol Mass of HBr = 209.0 g Known: Molar mass of HBr = 80.91…
Q: A solution formed by combining 10 mL of solution A and 40 mL of solution B. Find out the heat of…
A: Specific heat ( s) :- The amount of heat required to rise the temperature of 1 g of a substance…
Q: Using the balanced chemical reaction below, answer the following question: 2 HCI + Ba(ОН)2 BaCl2 + 2…
A: The number of moles of HCl in the solution of 216 mL of 0.21 M HCl is; nHCl=MHCl×VHCl=0.21 mol…
Q: How much heat is absorbed in the process of making 0.803 mol of CF4 from the following reaction? C…
A: Heat of formation, also called standard heat of formation, enthalpy of formation, or standard…
Q: How many grams of C̟H, liquid must decompose according to the following chemical equation to…
A:
Q: In the following reaction, how much heat (in kJ) is released when 2.09 moles of CH₄ are burned? CH₄…
A: The molar heat of combustion is the heat released when one mole of a substance is completely burned…
Q: How much heat will be released (∆H) if 0.4501 mol of NH₃ are mixed with 0.20 mol of O₂ in the…
A: This problem is based on the concept of limiting reagent.
Q: How many moles of H2 are required to produce -2501 kJ of heat in the following reaction? N2 (g) + 3…
A: Heat of reaction is defined as difference of energy of product and energy of Reactant. If heat of…
Q: How much heat will be released (∆H) if 1.20 mol of SrO is mixed with 0.951 mol of CO₂ in the…
A: Let x be the number of moles at CO2 required by 1.2 mol of SrO 1.2 mol of SrO requires 1.2 moles of…
Q: How much heat is absorbed in the process of making 0.899 mol of CF₄ from the following reaction? C…
A: For 1 mole of CF4,heat absorbed= 141.3 kJ
Q: .Excess heat energy liberated by an oxidation reaction iscalled the ___________.
A: Oxidation reaction is a reaction in which oxygen is added to the species. In terms of electron, in…
Q: How many moles of H₂ are required to produce -3001 kJ of heat in the following reaction? N₂ (g) + 3…
A:
Q: If the temperature of a solution raises, the reaction occuring (1.e., the system) is endothermic.…
A: Solution 1. If the temperature raises during the reaction, it means reaction releases heat ,…
Q: 7. When 0.63 g of solid LiCI (AHsoln = – 37.1 kJ/mol) dissolves in 5 L of water, how much heat is…
A: Answer :- Heat released (q) when 0.63 g LiCl dissolved in 5 L water = -552.8 (J) = -0.5528 (kJ)…
Q: How much heat will be released (∆H) if 0.1665 mol of NH₃ are mixed with 0.20 mol of O₂ in the…
A:
Q: In the following reaction, how much heat (in kJ) is released when 4.09 moles of CH₄ are burned? CH₄…
A: Methane reacts with oxygen to form carbon dioxide and water. The equation for the balanced chemical…
Q: How many grams of C6H, must decompose according to the following chemical equation to transfer 430…
A: The balanced equation is :- C6H6(l) ------> 3C2H2(g) Given, ∆H = 430 KJ
Q: How many moles of KCIO3 must be reacted according to the following balanced chemical reaction to…
A: The balanced reaction taking place is given as, => 2 KClO3 (s) -----> 2 KCl (s) + 3 O2 (g)…
Q: How many moles of H2 are required to produce -2151 kJ of heat in the following reaction? N2 (g) + 3…
A: The balanced reaction taking place is given as, =>
Q: In the following reaction, how many moles of CH₃OH are required to produce -6.696 × 10³ kJ of heat?…
A: The reaction given is 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g)…
Q: How many moles of H2 are required to produce -2501 KJ of heat in the following reaction? Heat…
A: Given Chemical reaction:
Q: How many moles of H₂ are required to produce -3451 kJ of heat in the following reaction? N₂ (g) + 3…
A: We have given that N2(g) + 3H2(g) -----> 2NH3(g) ∆H = -91.8KJ/mole
Q: Is the reverse reaction exothermic or endothermic?
A: Potential Energy Versus Reaction pathway is Given Nature of Reverse reaction Endothermic Or…
Q: How much heat (in kJ) is absorbed in the process of making 0.971 mol of CF4 from the following…
A: Carbon reacts with fluorine to form carbon tetrafluoride. The equation for the balanced chemical…
Q: In the following reaction, how many moles of CH3OH are required to produce -2191 kJ of heat? 2 CH3OH…
A: The balanced reaction taking place is given as,
Q: 1. What is the specific heat of a 50- gram metal initially at 200C which heated to 900 C with 2330 J…
A: Given: Initial temperature of metal = 200 oC Final temperature of metal = 900 oC Mass of metal = 50…
Q: How many moles of Hz are required to produce-3751 kJ of heat in the following reaction? N: (g) +3 H2…
A: For the given reaction: N2(g)+3H2(g)→2NH3(g) ∆H°=-91.8 kJ/mol That is 3 moles of H2 are required to…
Q: How many moles of KCIO, must be reacted according to the following balanced chemical reaction to…
A: 1) Write the balanced chemical equation. 2) Compare the number of moles of reactant with the amount…
Q: How many moles of KCIO. must be reacted according to the following balanced chemical reaction to…
A:
Q: In the following reaction, how many moles of CH;OH are required to produce -3.871 × 10³ kJ of heat?…
A: The given reaction is:2 CH3OH (l) + 3 O2 (g) → 2 CO2 (g) + 4 H2O (g)∆H° = -1280 kJ⇒ 1280 kJ of…
Q: During an experiment, a student calculated the heat of neutralization reaction to be - 52.8 kj/mol.…
A: Please find the attached images for the answer
Q: i don't know how to start this. we are supposed to get the heat of the reaction
A: Given
Q: In the following reaction, how much heat (in kJ) is released when 4.01 moles of CH₄ are burned? CH₄…
A:
Q: How much heat will be released (∆H) if 0.5659 mol of NH₃ are mixed with 0.20 mol of O₂ in the…
A: Given: The reaction is as follows, 4NH3(g)+O2(g) → 2N2H4(g)+2H2O(g) ∆H° = -286 kJ/mol Moles of…
Q: How many moles of KCIO, must be reacted according to the following balanced chemical reaction to…
A: Recall the given reaction 2KClO3s→2KCls + 3O2g ∆H=-89.4 kJWe have to find the moles of KClO3…
Q: How much heat is liberated at constant pressure if 0.938 g of calcium carbonate reacts with 73.2 mL…
A: Since molar mass of calcium carbonate = 100 g/mol Hence moles of calcium carbonate = mass / molar…
Q: A 4.81 g sample of an unknown salt (MM = 116.82 g/mol) is dissolved in 150.00 g water in a coffee…
A: Mass of unknown salt = 4.81 g Molar mass of unknown salt = 116.82 g / mol Moles of unknown salt…
Q: Answer: 14. 2H2 + 02 → 2H20 The heat of this reaction is - 42 kj. Is this reaction endothermic or…
A: Exothermic reaction is the reaction in which heat is released, whereas endothermic reactions are…
Q: 6. Calculate the heat change, in kJ, the following reaction:
A: Mass of PCl5 = 5.9217×1014ng = 5.9217×105g Molar mass of PCl5 = 208.24g/mol Moles of PCl5 =…
Heat released in reaction (q)
Step by step
Solved in 6 steps

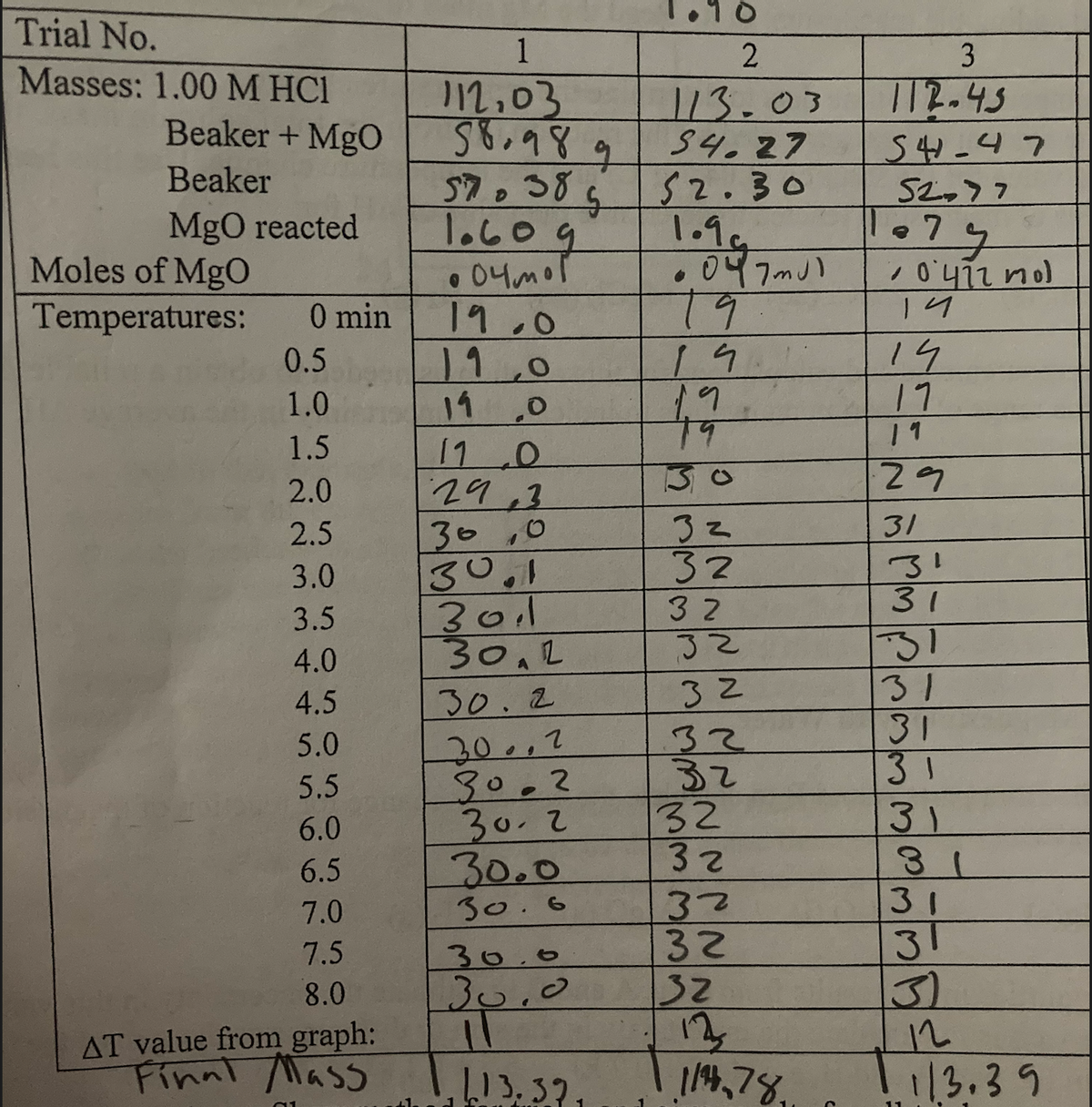
- Show all steps leading to the final answer po. Here’s a pdf file in accordance with the topic po: https://drive.google.com/file/d/1_FnDtXCrFKSol3RNWIG_9tNQ7IxgxD6t/view?usp=drivesdkQ) water hardness of each trial and average ppm with calculation, please. Hard Water Trial 1 Hard Water Trial 2 Hard Water Trial 3 Initial Syringe Reading 1.0ml 1.0ml 1.0ml Final Syringe Reading 0.88ml 0.84ml 0.85ml Volume of EDTA Consumed 0.12ml 0.16ml 0.15ml Water Hardness ppm CaCO3 Average ppmNH4+ {aq) + NO2(aq) -> N2(g) +2H2O{l} Data Initial [NH4+] Initial [NO2-] rate 1 0.0100 0.200 5.4 x10-7 2 0.0200 0.200 10.8x10-7 3 0.0400 0.200 21.5x10-7 4 0.200 0.0202 10.8x10-7 5 0.200 0.0404 21.6x10-7 6 0.200 0.0808 43.3x10-7 Find x,y,k
- What is ΔHsys for a reaction at 16.9 °C with ΔSsurr = -159 J mol-1 K-1 ? Express your answer in kJ mol-1 to at least two significant figures. (Please type answer no write by hend)3. a. Briefly explain why internal standardization method is useful inanalytical chemistry?b. Why does a response factor of an instrument’s detector need to becalculated?An analyst obtained the following data for the percent compound Z in triplicates (n=3) of an insecticide preparation: 7.47, 6.98, and 7.27. Calculate the 90% confidence limit for the mean of the data assuming that only information about the precision of the method is the precision for the three data points.
- A chemist obtained the following data for percent lindane in the triplicate analysis of an insecticide preparation: 7.23, 6.95, and 7.53%. Calculate the 90% confidence interval for the mean of the the three data, assuming that (a) the only information about the precision of the method is the precision for the three data. (b) on the basis of long experience with the method, it is believed that s---->σ lindane. (c) If s=0.28 is good estimate of σ, how many replicate measurement should be made in order for the mean for the analysis of sample to be within 0.2% of the true mean 90% of the time.Provide the remaining data missingWhat is ΔSsurr for a reaction at 28.6 °C with ΔHsys = 38.9 kJ mol-1 ? Express your answer in J mol-1 K-1 to at least two significant figures.
- can you answer, please? the experimental data are Reference: https://www.youtube.com/watch?v=OOXRkycKEOc&feature=youtu.be and https://www.youtube.com/watch?v=3wLJLm0QLpg&feature=youtu.bePV = nRT. The pressure is 0.9912atm. V = 50.5 mL. T = 21.5°C and n = 0.00200mol. Calculate R from this data in Latm/molK.Initial temp: 19.06 C trial 1: 0.5 M concentration trial 2 : 1.0 M concentration 200 mL of HCl and 200 mL of NaOH are combined in an insulated container. HCl = 0.5 mol/ml x 200 x 10^-3 ml = 0.1 mol NaOH = 0.5 mol/ml x 200 x 10^-3 ml= 0.1 mol H+(aq) + OH-(aq) - - -> H2O (l) ∆H H++ = 0, ∆H OH−− = -230 kJ/mol, ∆H H2O = -286 kJ/mol ∆H reactants = ∆H H++ + ∆H OH−− = -230 + 0 = -230 kJ/mol ∆H reactants = -286 kJ/mol - ( -230 kJ/mol) = -56 kJ/mol For each trial, calculate how much energy is released during this reaction.

