Heavy metal sulfides like CdS and PbS are often significant components of mine tailings (the piles of waste and low-grade ores discarded by a mining operation). While these sulfides are quite insoluble in water (Ksp of CdS = 1 E -27, Ksp of PbS = 3 E -28), over the years rainwater and snowmelt can slowly dissolve small amounts of these heavy metals, washing them into streams and other natural waters. If these mine tailings are located in an area that is impacted by acid rain, would you expect heavy metal dissolution into natural waters to increase or decrease? Use chemical reactions to justify your answer.
Heavy metal sulfides like CdS and PbS are often significant components of mine tailings (the piles of waste and low-grade ores discarded by a mining operation). While these sulfides are quite insoluble in water (Ksp of CdS = 1 E -27, Ksp of PbS = 3 E -28), over the years rainwater and snowmelt can slowly dissolve small amounts of these heavy metals, washing them into streams and other natural waters. If these mine tailings are located in an area that is impacted by acid rain, would you expect heavy metal dissolution into natural waters to increase or decrease? Use chemical reactions to justify your answer.
Chapter20: The Representative Elements
Section: Chapter Questions
Problem 54E
Related questions
Question
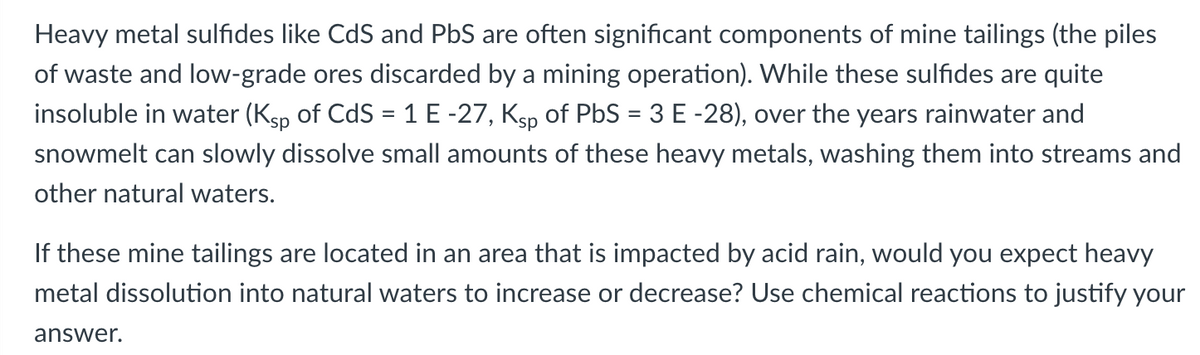
Transcribed Image Text:Heavy metal sulfides like CdS and PbS are often significant components of mine tailings (the piles
of waste and low-grade ores discarded by a mining operation). While these sulfides are quite
insoluble in water (Ksp of CdS = 1 E -27, Ksp of PbS = 3 E -28), over the years rainwater and
snowmelt can slowly dissolve small amounts of these heavy metals, washing them into streams and
other natural waters.
If these mine tailings are located in an area that is impacted by acid rain, would you expect heavy
metal dissolution into natural waters to increase or decrease? Use chemical reactions to justify your
answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning