Hello! Can you please explain what the difference between the four orbitals are? I know that there are four- s,d,p,f, but I am having trouble understanding differentiating the four.
Hello! Can you please explain what the difference between the four orbitals are? I know that there are four- s,d,p,f, but I am having trouble understanding differentiating the four.
The region where electrons are found is said to be an Orbital. There are four orbitals namely, s, p, d and f.
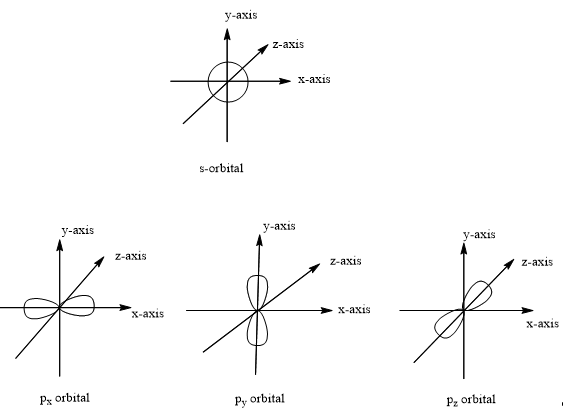
The s-orbital is spherical and symmetric. The s-orbital is the smallest orbital and hold maximum of 2 electrons. As the energy levels increases, the size of the orbital increases and electrons are moved away from nucleus. The order of size of orbitals is 1s<2s<3s…
In 1s-orbital, no nodes are present. In 2s-orbital, one node, in 3s-orbital, 2 nodes and so on are present.
The p-orbital is dumbbell shaped. The electrons enter into p-orbital after 1s and 2s orbitals got filled. The p-orbital holds a maximum of 6 electrons. At any energy level, three equivalent p-orbitals are present pointing right angles to each other in x, y and z directions. They are px, py and pz.
The shapes of s and p-orbitals can be as follows:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









