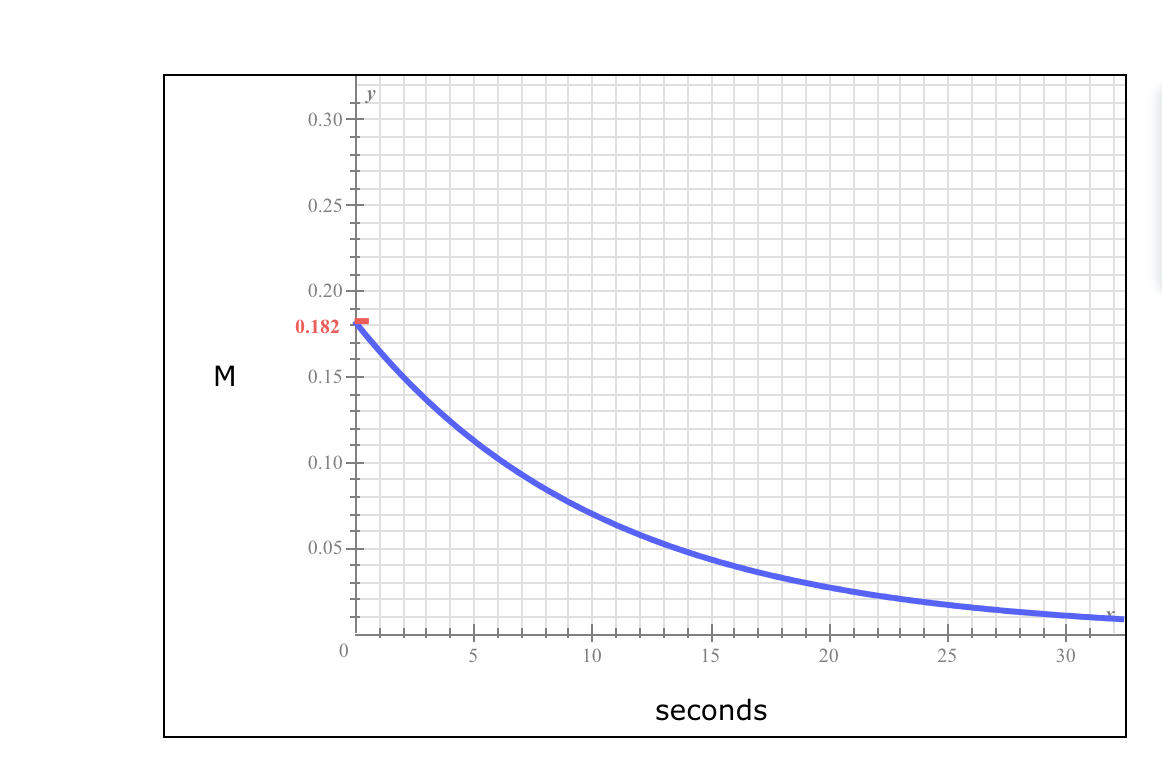
Here is a graph of the molarity of bromine Br2 in a reaction vessel during a certain chemical reaction. If Br2 is being created or destroyed, what is the rate at which it is being created or destroyed 10 seconds after the reaction starts? Round your answer to 2 significant digits. If Br2 is being created or destroyed, what is the average rate at which it is being created or destroyed during the first 10 seconds of the reaction? Round to 2 significant digits.
Here is a graph of the molarity of bromine Br2 in a reaction vessel during a certain chemical reaction. If Br2 is being created or destroyed, what is the rate at which it is being created or destroyed 10 seconds after the reaction starts? Round your answer to 2 significant digits. If Br2 is being created or destroyed, what is the average rate at which it is being created or destroyed during the first 10 seconds of the reaction? Round to 2 significant digits.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
ChapterL: Let's Review
SectionL.2: Making Measurements: Precision, Accuracy, Experimental Error, And Standard Deviation
Problem 1RC
Related questions
Question
Here is a graph of the molarity of bromine Br2 in a reaction vessel during a certain
| If
Br2
|
|
| Round your answer to
2
|
|
| If
Br2
|
|
| Round to
2
|
Transcribed Image Text:M
0.30-
0.25-
0.20-
0.182
0.15+
0.10+
0.05-
0
y
5
10
15
seconds
20
25
30
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step 1: Given data
VIEWStep 2: Determining whether Br2 is being created or destroyed
VIEWStep 3: Determining the rate at which Br2 is being destroyed 10 seconds after the reaction starts
VIEWStep 4: Determining average rate at which Br2 is being destroyed during the first 10 seconds of the reaction
VIEWSolution
VIEWStep by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning