High in the mountains, an explorer notes that the water for tea is boiling vigorously at a temperature of 79 °C. Use the data in the table below to estimate the atmospheric pressure at the altitude of the camp. Estimate AHyap for water between 79 and 81 °C. Atmospheric pressure = atm AHvap kJ/mol Vapor Pressure of Water at Various Temperatures. T°C P atm 77 78 79 80 81 0.413 0.431 0.449 0.467 0.486 82 0.506 83 0.527 84 0.548 85 0.571 86 0.593
High in the mountains, an explorer notes that the water for tea is boiling vigorously at a temperature of 79 °C. Use the data in the table below to estimate the atmospheric pressure at the altitude of the camp. Estimate AHyap for water between 79 and 81 °C. Atmospheric pressure = atm AHvap kJ/mol Vapor Pressure of Water at Various Temperatures. T°C P atm 77 78 79 80 81 0.413 0.431 0.449 0.467 0.486 82 0.506 83 0.527 84 0.548 85 0.571 86 0.593
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 24PS: Methane (CH4) cannot be liquefied at room temperature, no matter how high the pressure. Propane...
Related questions
Question
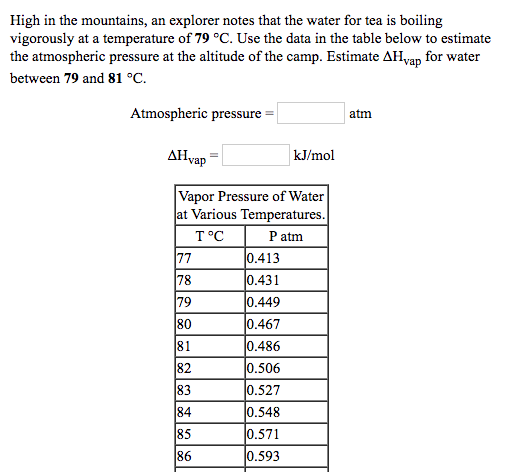
Transcribed Image Text:High in the mountains, an explorer notes that the water for tea is boiling
vigorously at a temperature of 79 °C. Use the data in the table below to estimate
the atmospheric pressure at the altitude of the camp. Estimate AHyap for water
between 79 and 81 °C.
Atmospheric pressure =
atm
AHvap
kJ/mol
Vapor Pressure of Water
at Various Temperatures.
T°C
P atm
77
78
79
80
81
0.413
0.431
0.449
0.467
0.486
82
0.506
83
0.527
84
0.548
85
0.571
86
0.593
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning