Q: Have each group member select one of the cubic crystalline lattices. Learn everything you can about ...
A:
Q: The nearest star to our sun is Proxima Centauri, at a distance of 4.3 light-years from the sun. A li...
A: Given, The distance in light year = 4.3 light year
Q: Phosgene (Cl2CO) is a poisonous gas used as a chemical weapon during World War I. It is a potential ...
A: Given; Phosgene Cl2CO is a poisonous gas that was used as a chemical weapon during World War I. It i...
Q: determine the enthalpy value in the reaction of C2H5OH (I) from the following equations ...
A: The reactions given are 1) C2H5OH (l) + 3 O2 (g) → 2 CO2 (g) + 3 H2O (l) ...
Q: Sketch the phase diagram for carbon dioxide. If you have carbon dioxide at 1.0 atm and 25 °C, could ...
A: Phase diagram for carbon dioxide,
Q: Chemistry Question
A: The vapour pressure of any liquid at its normal boiling point is 1 atm. Hence the vapour pressure of...
Q: Can you please explain how, not given the products, to know what the products will be from the react...
A:
Q: According to the quantum-mechanical model for the hydrogen atom, which electron transition produces ...
A: The energy of a photon can be written as follow: E = hc /wavelength Thus, the lower the energy will ...
Q: Which of the following statements concerning double covalent bonds is correct?A) they are found only...
A: Double covalent bond means that there is a double bond between the atoms.
Q: What is the bond angle of SeI2
A: The number of valence electron in centre atom Se is 6 And in I, the number of valence electron is 7 ...
Q: "Substitution is a reaction in which an atom or a group of atoms isreplaced by another atom or group...
A: Substitution is a reaction in which an atom or a group of atoms is replaced by another atom or group...
Q: Which of the following can hydrogen bond with water? A. Br2 B. CH3CH2CH3 ...
A: For any molecule to form H bonds with water, it should be either acting as hydrogen bond donor or hy...
Q: A dioxin-contaminated water source contains 0.085% dioxin by mass. How much dioxin is present in 2.5...
A:
Q: Solubility of terazosin? With illustration
A:
Q: The equilibrium constant for the conversion of the axial to the equatorial conformation of methoxycy...
A: a) If the equilibrium constant, K>1 then equilibrium favors towards the product. So, according t...
Q: Draw an energy diagram for a reaction in which the products are higher in energy than the starting m...
A: The energy diagram of a reaction is the graph plotted between potential energy vs reaction progress....
Q: A substance has a heat of vaporization of ΔHvap and a heat of fusion of ΔHfus. Express the heat of s...
A: The process where solid turns into solution (liquid) is considered as "fusion reaction" and the heat...
Q: In the basic medium, write the compound that gives 1 mol of acetone and 1 mol of acetic acid when he...
A: The oxidative cleavage of alkenes is performed in the presence of potassium permanganate. The double...
Q: II ded for this question. Write a balanced nuclear equation for the following: The nuclide thallium-...
A: Beta decay refers to the radioactive decay in which a beta particle is emitted from an atomic nucleu...
Q: Trabectedin, shown in a ball-and-stick model on the cover of this text, isan anticancer drug sold un...
A: SOLUTION: Step 1: Trabectedin is composed of three tetrahydroisoquinoline moieties, eight rings incl...
Q: In an electrochemical cell a metal anode Lost 0.124 g well total volume of 0.04057 L of hydrogen gas...
A: barometric pressure = 766.9 mm Hg vapour pressure of water = 23.8 mm Hg partial pressure of H2=766.9...
Q: An X-ray beam with l = 154 pm incident on the surface of a crystal produced a maximum reflection at ...
A: Given: An X-ray beam with, λ = 154 pm Ө = 28.3o n = 1
Q: What is the trend in the size of the band gap as you move down the column of the group 4A elements?
A: Band-In molecular orbitals of an atom the set of closely discrete energy levels is called as band. V...
Q: Donepezil is the drug used to treat Alzheimer's disease. Predict the approximate bond angle, d in Do...
A: The structure of donepezil is shown below:
Q: Explain Types of Energy?
A: SOLUTION: Step 1: Energy is defined as the ability to do work. Energy comes in various forms. Energy...
Q: Balance the chemical equation for the combustion of ethanol _CH3CH2OH(g) +_02(g)→CO2(g) +_H2O() _CO2...
A: Combustion reactions are those in which the organic compound reacts with the atmospheric oxygen to g...
Q: Chemistry Question
A: Given, electricity generated in South Africa per year = 234.5 x 109 kWh = 234.5 x 109 x 10-3 MWh =...
Q: A solution of 49.0% H2SO4 by mass has a density of 1.39 g/cm3 at 293 K. A 25.0-cm3 sample of this so...
A: In 100 gm of solution, 49 gm is H2SO4 . Volume of solution = Mass / Density ...
Q: Suppose that in an alternate universe, the possible values of l are the integer values from 0 to n (...
A:
Q: Identify each solid as molecular, ionic, or atomic.a. Ar(s)b. H2O(s)c. K2O(s)d. Fe(s)
A: Molecule refers to the group of atoms present as a whole that can take part in a chemical reaction. ...
Q: Which of the following is polar (possesses a dipole moment)? A. C6H6 B. C...
A:
Q: Explain the significance of the slope and intercept of an Arrhenius plot.
A: The Arrhenius equation is shown below. where, K is the rate constant of the equation A is the Arrhe...
Q: Have each group member select and study a material from the section on ceramics, cement, and glass. ...
A:
Q: Distillation is a method of purification based on successive separations and recondensations of vapo...
A:
Q: In Section 8.6, we estimated the effective nuclear charge on beryllium’s valence electrons to be sli...
A:
Q: A proton in a linear accelerator has a de Broglie wavelength of 122 pm. What is the speed of the pro...
A: Given: De Broglie wavelength of proton is 122 pm Formula used: De Broglie wavelength λ = h/mv where,...
Q: Octane and nonane are liquids which are components of gasoline. Their vapor pressures at 25°C are 13...
A: "Raoult’s law" is mainly applicable for "ideal solutions" only. If any solution follows "Raoult’s la...
Q: What volume of H2 gas forms at 1.0 atm and 25 degrees Celsius when 55.0mL of 5.0M H2SO4reacts with 6...
A: The reaction taking place will be Al (s) + H2SO4 (aq) -------> Al2(SO4)3 (aq) + H2 (g) Balancing...
Q: A 0.500 g sample of the explosive TNT (C7H5N3O6) is completely burned in a bomb calorimetercontainin...
A:
Q: Hello the aanswer of this question is wrong in the second part in your website. It is not 2.55 ...
A:
Q: Provide major product and mechanism for the following reaction *most simple
A: Since the alkyne group is electron rich. Hence it will attack on Cl2 and form a intermediate with ri...
Q: Why do the rows in the periodic table get progressively longer as you move down the table? For examp...
A:
Q: Glycine has the zwitterion structure shown. draw the structure and give the net charge of glycine th...
A: a)
Q: Which compound do you expect to be soluble in octane (C8H18)?a) CH3OH b) CBr4 c) H2O d) NH3
A: The compound expected to be soluble in octane (C8H18) has to be determined
Q: Scuba divers breathing air at increased pressure can suffer from oxygen toxicity—too much oxygen in ...
A: GIVEN: Scuba divers breathing air at increased pressure can suffer from oxygen toxicity—too much oxy...
Q: Give an example of metal which(i) is a liquid at room temperature(ii) can be easily cut with knife(i...
A: Metals like Gallium, Mercury are almost in liquid form at room temperature. The very soft metal whic...
Q: A first-order reaction has rate constants of 4.6 x 10-2 s-1 and 5.6 x 10-2 s-1 at 0°C and 20.°C, res...
A:
Q: A solution contains 4.08 g of chloroform (CHCl3) and 9.29 g of acetone (CH3COCH3). The vapor pressur...
A: SOLUTION: Step 1: Atomic weight of acetone =58.08 g/mol. Atomic weight of chloroform = 119.38 g/mol....
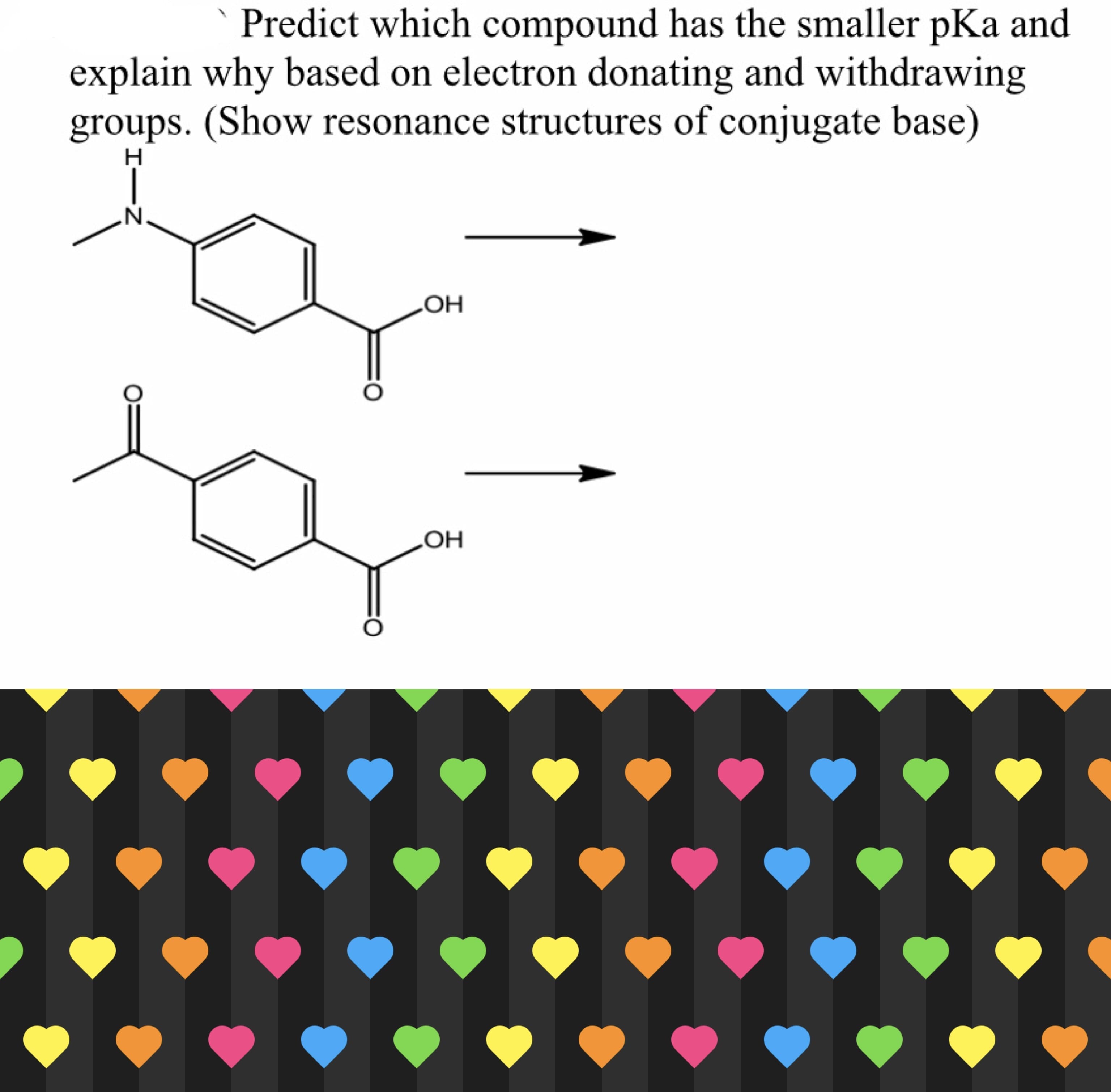
SOLUTION:
Step 1:
(a) Acidity of a compound arise when it can lose proton in aqueous solution. Here, due to +M effect of CH3NH- group it pushes the electron density towards the -COOH group through the benzene ring through resonance. So, the electron density on -OH bond of -COOH group is high. So, this proto is not easily available in aqueous solution. The compound will be less acidic and have high pKa value.

Step by step
Solved in 2 steps with 2 images


