Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.6PAE: Use the web to research the amount of PVC polymer produced annually in the United States. What are...
Related questions
Question
Answer the following question about curcumin, a yellow pigment
isolated from turmeric, a tropical perennial in the ginger family and a
principal ingredient in curry powder.
Most enols, compounds that contain a hydroxy group bonded to a C=C, are unstable and tautomerize to carbonyl groups. Draw the keto form of the enol of curcumin, and explain why the enol is more stable than many other enols.
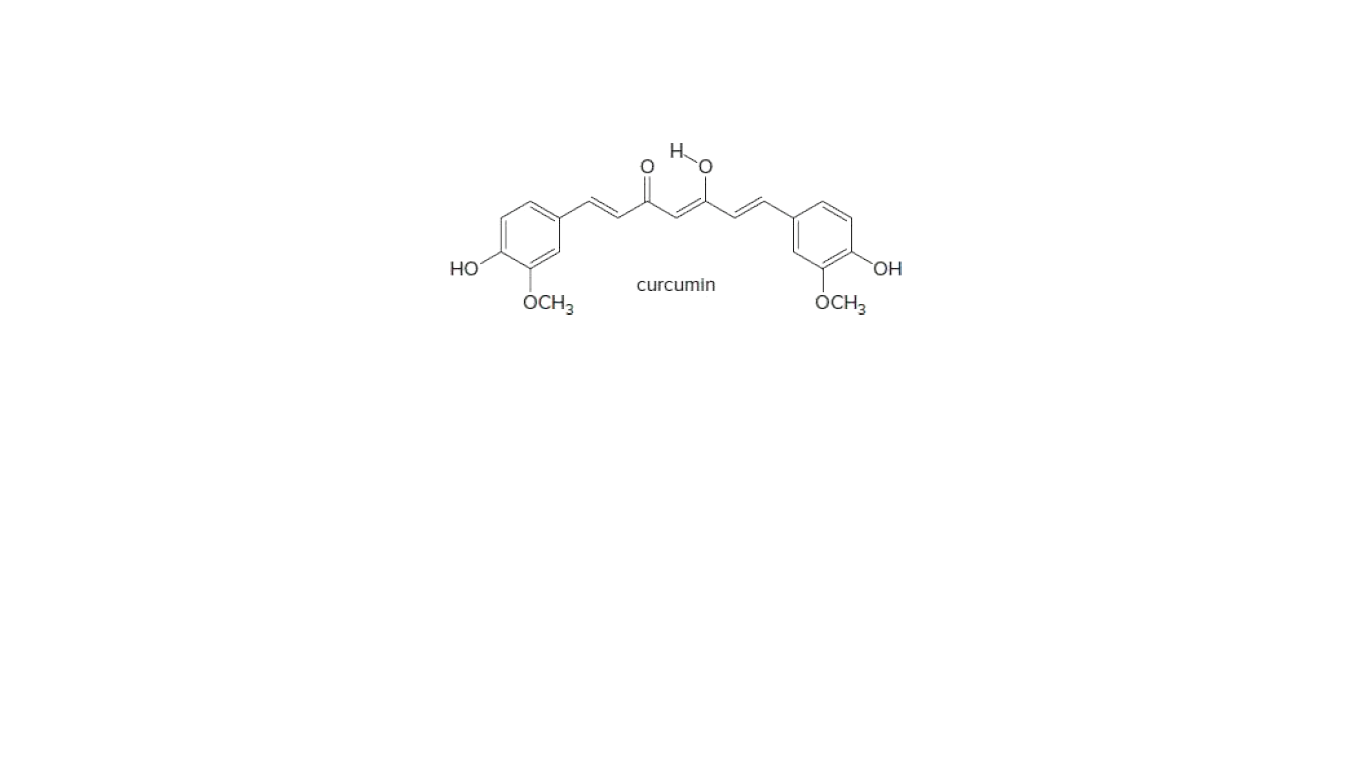
Transcribed Image Text:HO
HO.
curcumin
ÓCH3
ÓCH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning