Homologous Series: Relating Intermolecular Forces, Boiling Points, and Molecular Formula Rank from highest to lowest boiling point. To rank items as equivalent, overlap them. > View Available Hint(s) Reset CH3 CH,CH2CH2CH,CH2CH3 CH,CH,CH,CH3 CH3CH2CCH2CH3 CH, CH,CH2CH2CH,CH2CH3 CH3 Lowest boiling Highest boiling point O The correct ranking cannot be determined. Submit
Homologous Series: Relating Intermolecular Forces, Boiling Points, and Molecular Formula Rank from highest to lowest boiling point. To rank items as equivalent, overlap them. > View Available Hint(s) Reset CH3 CH,CH2CH2CH,CH2CH3 CH,CH,CH,CH3 CH3CH2CCH2CH3 CH, CH,CH2CH2CH,CH2CH3 CH3 Lowest boiling Highest boiling point O The correct ranking cannot be determined. Submit
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter14: Liquids And Solids
Section: Chapter Questions
Problem 38QAP: Two molecules that contain the same number of each kind of atom but [hat have different molecular...
Related questions
Question
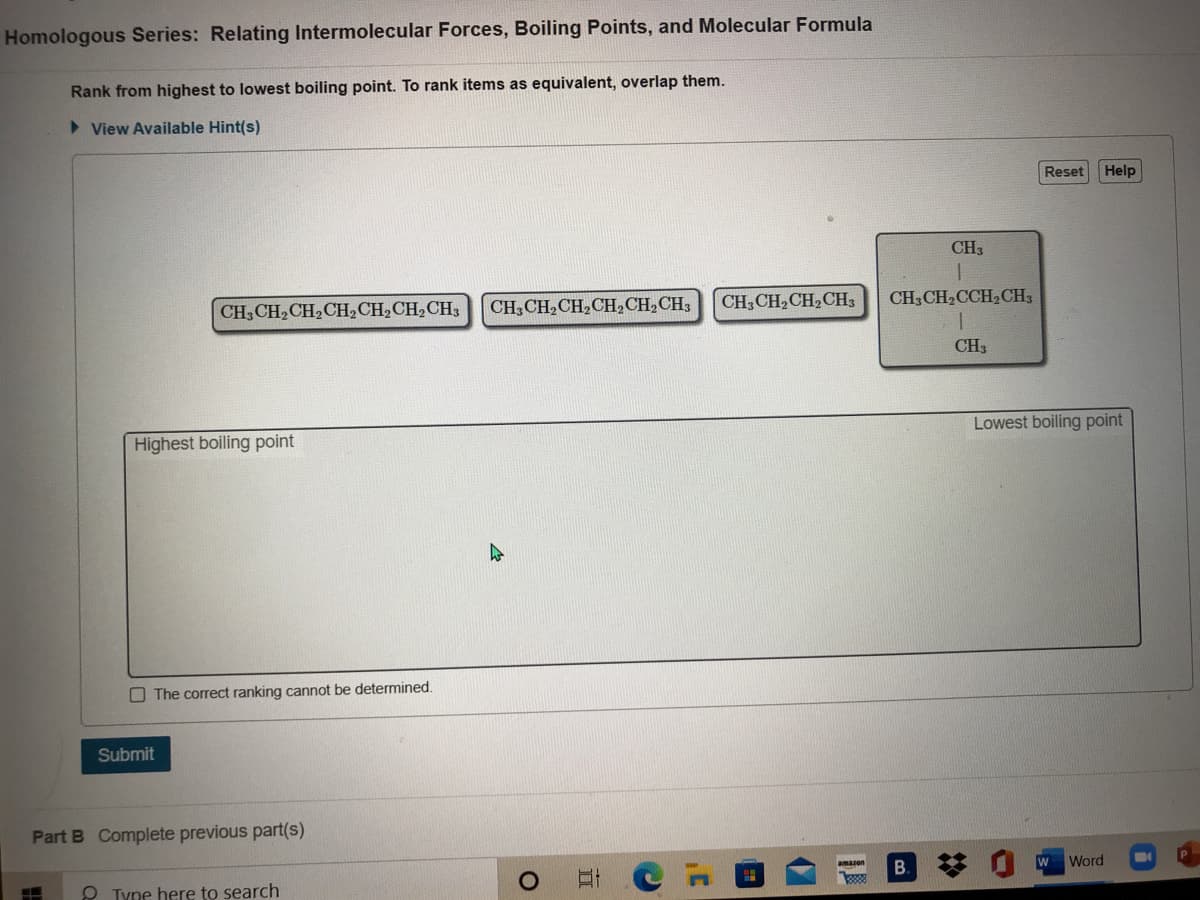
Transcribed Image Text:Homologous Series: Relating Intermolecular Forces, Boiling Points, and Molecular Formula
Rank from highest to lowest boiling point. To rank items as equivalent, overlap them.
> View Available Hint(s)
Reset
Help
CH3
CH,CH,CH2CH2CH,CH2CH3
CH3CH2CH,CH2CH,CH3
CH,CH2CH2CH3
CH,CH,CCH2CH3
CH3
Highest boiling point
Lowest boiling point
O The correct ranking cannot be determined.
Submit
Part B Complete previous part(s)
amazon
B.
Word
W
O Tyne here to search
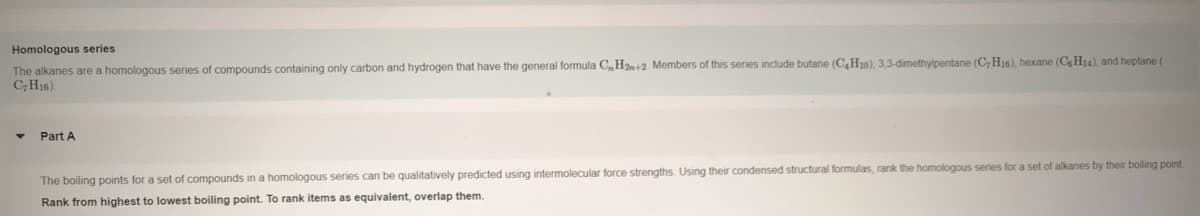
Transcribed Image Text:Homologous series
The alkanes are a homologous series of compounds containing only carbon and hydrogen that have the general formula C,H2n+2. Members of this series include butane (C,H10), 3,3-dimethylpentane (C,H16), hexane (C H14), and heptane (
CH16).
Part A
The boiling points for a set of compounds in a homologous series can be qualitatively predicted using intermolecular force strengths. Using their condensed structural formulas, rank the homologous series for a set of alkanes by their boiling point
Rank from highest to lowest boiling point. To rank items as equivalent, overlap them.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning