How did the change of stress (adding or removing reactants or products) cause a shift in the equilibrium system of the solutions (in which direction)
How did the change of stress (adding or removing reactants or products) cause a shift in the equilibrium system of the solutions (in which direction)
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU6: Showtime: Reversible Reactions And Chemical Equilibrium
SectionU6.7: How Colorful: Applying Le Chatelier's Principle
Problem 8E
Related questions
Question
How did the change of stress (adding or removing reactants or products) cause a shift in the equilibrium system of the solutions (in which direction) use an example. Is trial one as an example

Transcribed Image Text:ABC
CONTROL
When the amounts of Co(H₂O)6²+ and COCI4²-
are approximately equal in the test tube, the
solution is purple, as in control test tube B.
CONTINUE
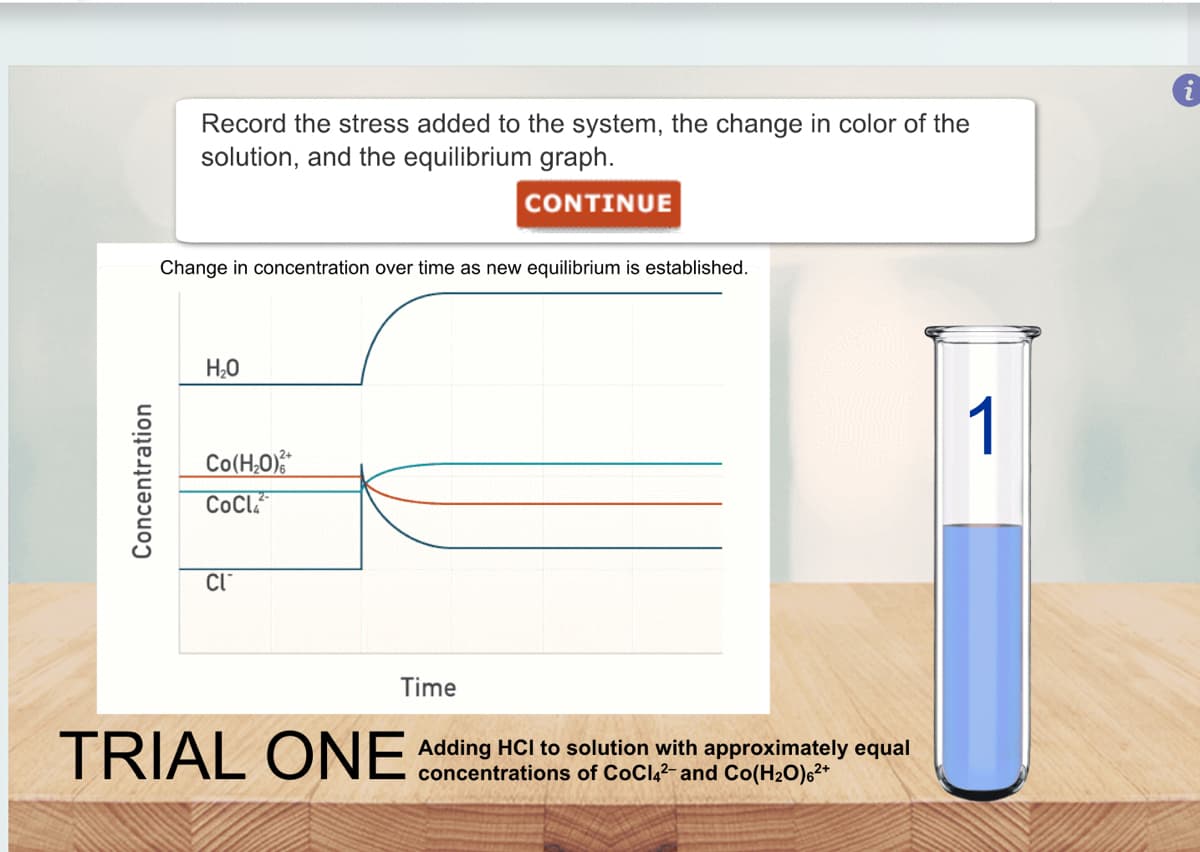
Transcribed Image Text:Concentration
Record the stress added to the system, the change in color of the
solution, and the equilibrium graph.
CONTINUE
Change in concentration over time as new equilibrium is established.
H₂O
Co(H,O)%
CoCL
CI™
Time
TRIAL ONE Adding HCI to solution with approximately equal
of CoCl4²- and Co(H₂O)6²+
1
i
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning