How do intermolecular forces relate to the physical properties of substances? (ou will examine several physical properties of three liquids: water, ethanol, and yclohexane. The chemical structure of each chemical is in the table below. Water Ethanol Cyclohexane H- -OH H. H 1. Solubility If two liquids mix together (no layer forms), they are soluble. Try different combinations of the three liquids in a small test tube to determine which are soluble in each other. Use approximately 5 mL of each liquid. Dispose of your combinations in the "IMF Discovery" waste beaker. a. Record your observations. b. Describe the polarity (polar or non-polar) of each molecule. Which polarities mix and which do not? Use the concept of intermolecular forces to explain your observations. 2. Surface Tension
How do intermolecular forces relate to the physical properties of substances? (ou will examine several physical properties of three liquids: water, ethanol, and yclohexane. The chemical structure of each chemical is in the table below. Water Ethanol Cyclohexane H- -OH H. H 1. Solubility If two liquids mix together (no layer forms), they are soluble. Try different combinations of the three liquids in a small test tube to determine which are soluble in each other. Use approximately 5 mL of each liquid. Dispose of your combinations in the "IMF Discovery" waste beaker. a. Record your observations. b. Describe the polarity (polar or non-polar) of each molecule. Which polarities mix and which do not? Use the concept of intermolecular forces to explain your observations. 2. Surface Tension
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter12: Solutions
Section: Chapter Questions
Problem 12.25QP: Consider two hypothetical pure substances, AB(s) and XY(s). When equal molar amounts of these...
Related questions
Question
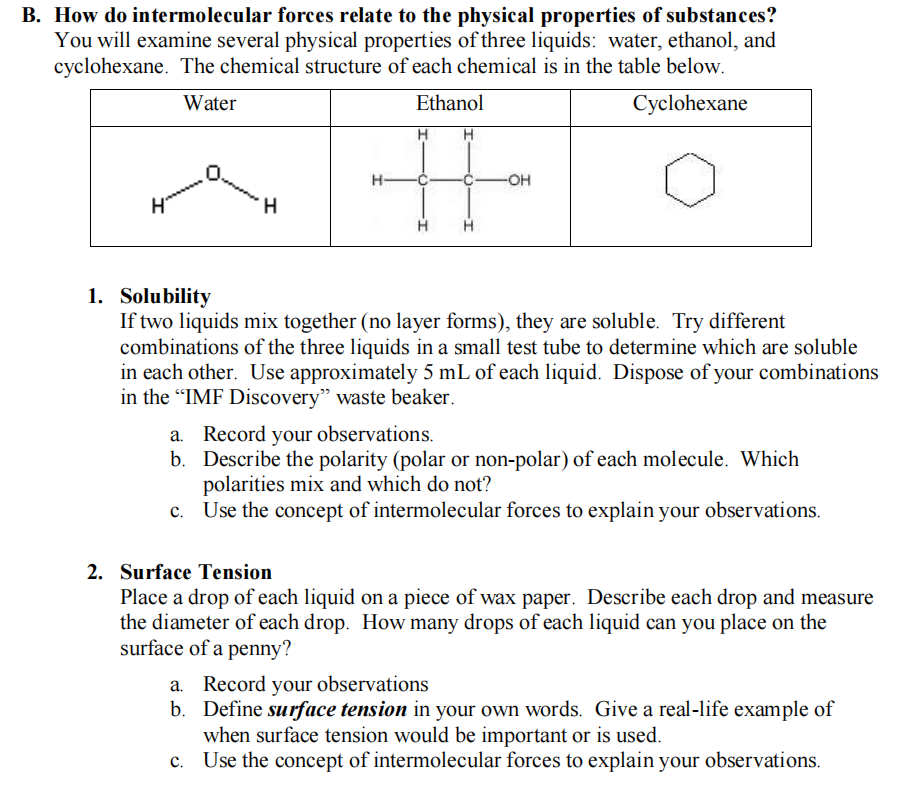
Transcribed Image Text:B. How do intermolecular forces relate to the physical properties of substances?
You will examine several physical properties of three liquids: water, ethanol, and
cyclohexane. The chemical structure of each chemical is in the table below.
Water
Ethanol
Cyclohexane
%23
H-
-OH-
ymmm
1. Solubility
If two liquids mix together (no layer forms), they are soluble. Try different
combinations of the three liquids in a small test tube to determine which are soluble
in each other. Use approximately 5 mL of each liquid. Dispose of your combinations
in the "IMF Discovery" waste beaker.
a. Record your observations.
b. Describe the polarity (polar or non-polar) of each molecule. Which
polarities mix and which do not?
c. Use the concept of intermolecular forces to explain your observations.
2. Surface Tension
Place a drop of each liquid on a piece of wax paper. Describe each drop and measure
the diameter of each drop. How many drops of each liquid can you place on the
surface of a penny?
a. Record your observations
b. Define surface tension in your own words. Give a real-life example of
when surface tension would be important or is used.
c. Use the concept of intermolecular forces to explain your observations.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning