Chapter3: Using Spreadsheets In Analytical Chemistry
Section: Chapter Questions
Problem 3.4QAP
Related questions
Question
How does the -1 go away? How come 2 still stays positive ? Even after switching sides?

Expert Solution
Step 1
The calcium iodide is a ionic compound as this is formed by the reaction of metal and non-metal.
So, from periodic table, Calcium have +2 charge and iodide have -1 charge, But any compound is neutral i.e do not have any charge on them.
Step 2
To make their charge-neutral, mention crisscrosses their charges to the subscript of elements.
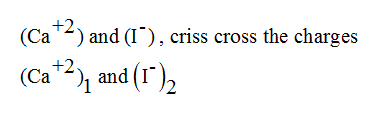
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

