How does the pKe of the conjugate acid of benzylamine (CgH5CH,NH2) compare to the pk,'s of the conjugate acids of cyclohexanamine (10.7) and aniline (4.6)? Explain your choice.
How does the pKe of the conjugate acid of benzylamine (CgH5CH,NH2) compare to the pk,'s of the conjugate acids of cyclohexanamine (10.7) and aniline (4.6)? Explain your choice.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter23: Amines
Section: Chapter Questions
Problem 23.26P: The pKa, of the conjugate acid of morpholine is 8.33. (a) Calculate the ratio of morpholine to...
Related questions
Question
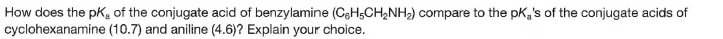
Transcribed Image Text:How does the pKe of the conjugate acid of benzylamine (CgH5CH,NH2) compare to the pk,'s of the conjugate acids of
cyclohexanamine (10.7) and aniline (4.6)? Explain your choice.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
