How long will it take to produce 78 g of Al metal by the reduction of Al3+* in an electrolytic cell with a current of 2.0 A? Multiple Choice 10 - 1012, 0.01s 420 s 13 h 116 h
How long will it take to produce 78 g of Al metal by the reduction of Al3+* in an electrolytic cell with a current of 2.0 A? Multiple Choice 10 - 1012, 0.01s 420 s 13 h 116 h
Glencoe Physics: Principles and Problems, Student Edition
1st Edition
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Paul W. Zitzewitz
Chapter20: Static Electricity
Section: Chapter Questions
Problem 4STP
Related questions
Question
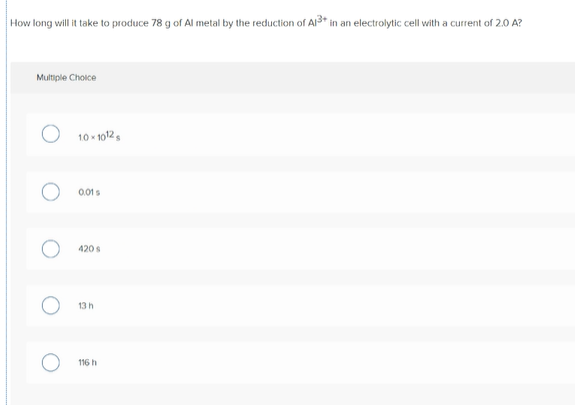
Transcribed Image Text:How long will it take to produce 78 g of Al metal by the reduction of Al3+* in an electrolytic cell with a current of 2.0 A?
Multiple Choice
10 - 1012,
0.01s
420 s
13 h
116 h
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning