Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter11: Solutions And Colloids
Section: Chapter Questions
Problem 50E: A sample of an organic compound (a nonelectrolyte) weighing 1.35 g lowered the freezing point of...
Related questions
Question
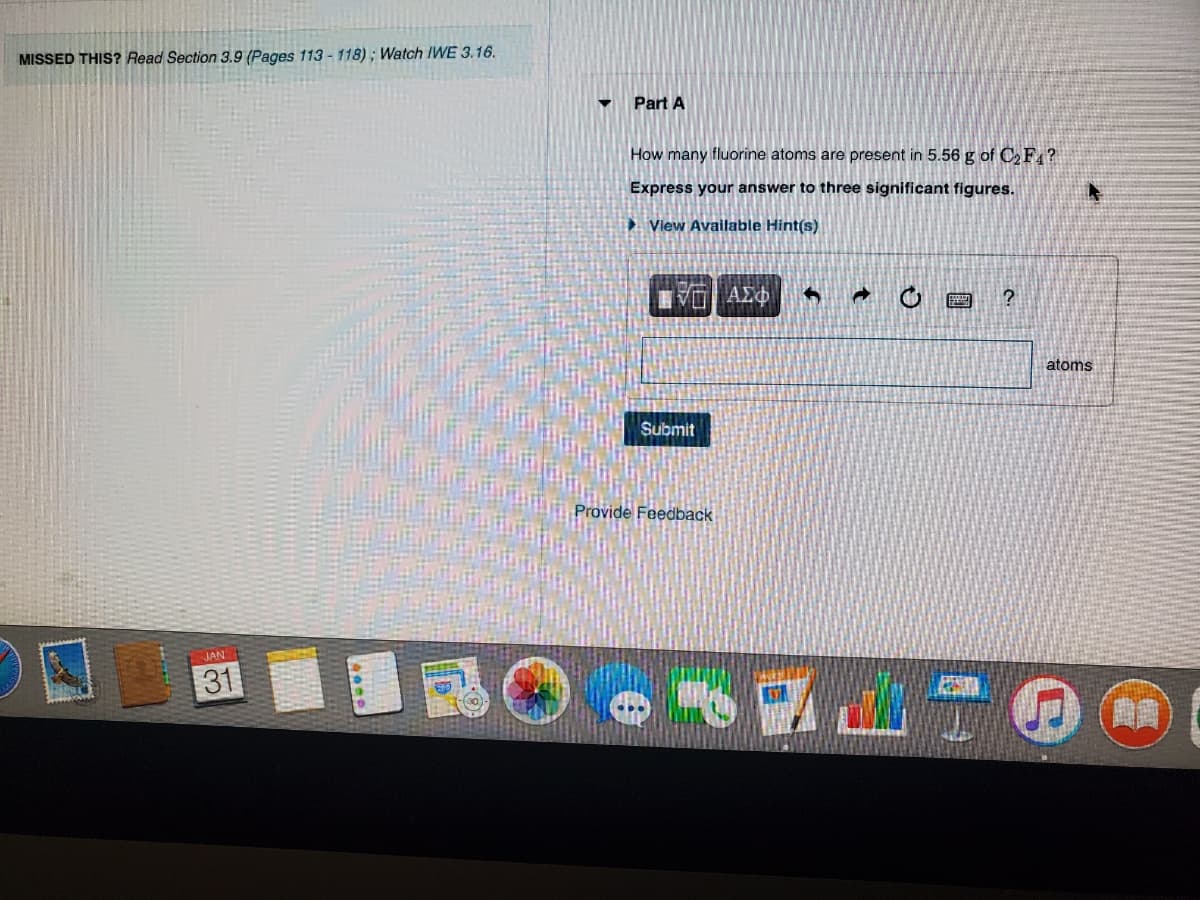
Transcribed Image Text:MISSED THIS? Read Section 3.9 (Pages 113-118); Watch IWE 3.16.
Part A
How many fluorine atoms are present in 5.56 g of CF ?
Express your answer to three significant figures.
► Vlew Available Hint(s)
atoms
Submit
Provide Feedback
JAN
31
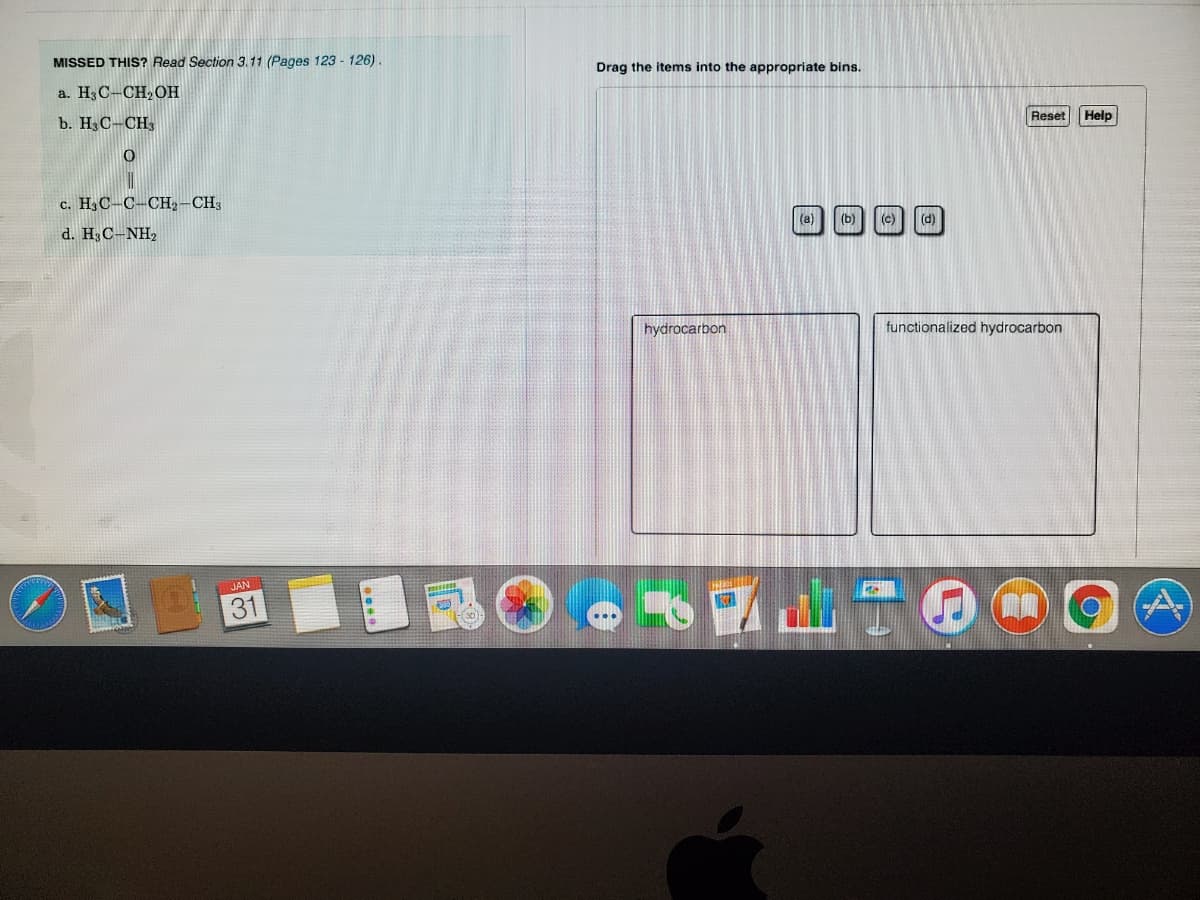
Transcribed Image Text:MISSED THIS? Read Section 3.11 (Pages 123 - 126).
Drag the items into the appropriate bins.
a. H3C-CH, OH
Reset
Help
b. H3C-CH3
c. H3C-C-CH,-CH3
(a)
(b)
(e)
(d)
d. H C-NH,
hydrocarbon
functionalized hydrocarbon
JAN
31
Expert Solution
Step 1
“Since you have asked multiple questions, we will solve the first question for you. If you want any specific question to be solved, then please specify the question number or post only that question.”
According to the mole concept, in terms of mass, the amount of substance in moles is equal to the ratio of its mass to the molar mass.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
