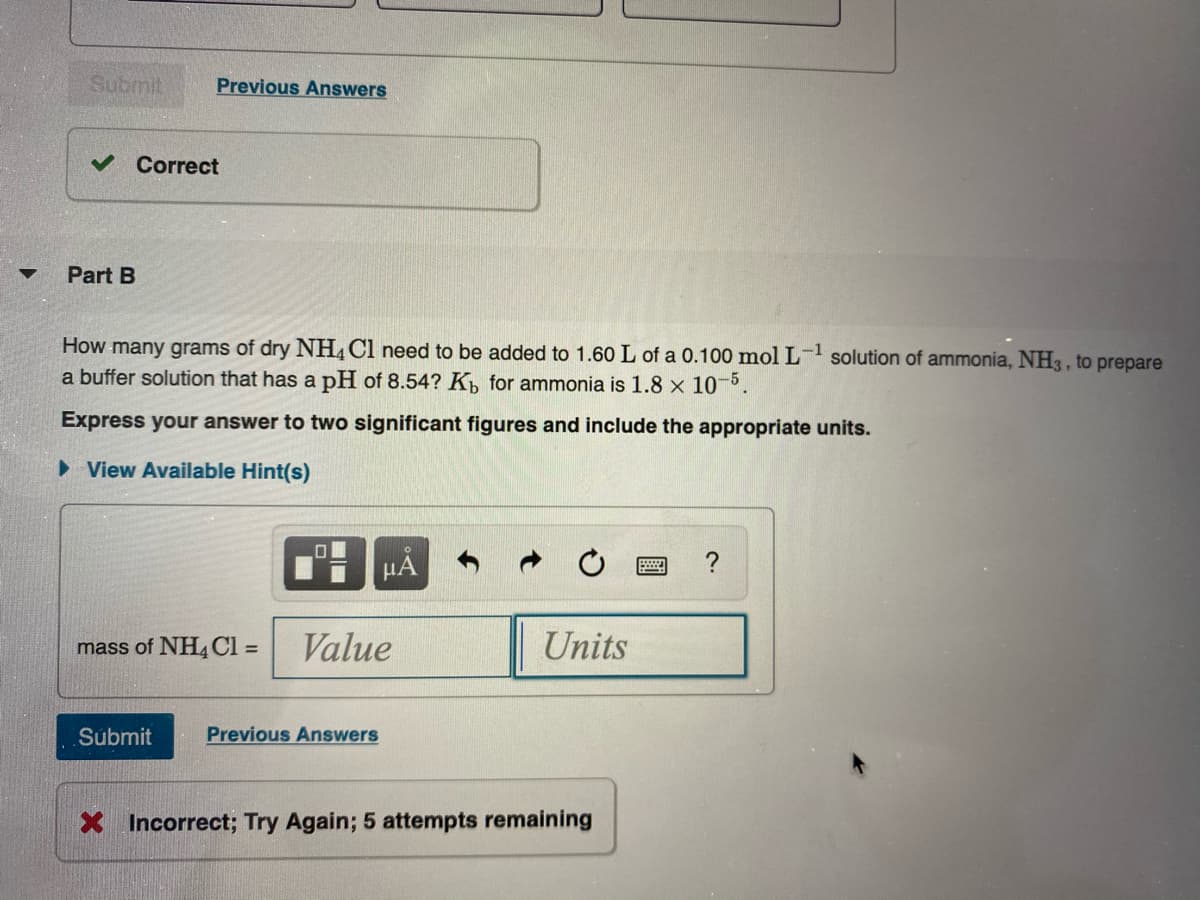
How many grams of dry NH,Cl need to be added to 1.60 L of a 0.100 mol L- solution of ammonia, NH3 , to prepare a buffer solution that has a pH of 8.54? K, for ammonia is 1.8 x 10. Express your answer to two significant figures and include the appropriate units.
How many grams of dry NH,Cl need to be added to 1.60 L of a 0.100 mol L- solution of ammonia, NH3 , to prepare a buffer solution that has a pH of 8.54? K, for ammonia is 1.8 x 10. Express your answer to two significant figures and include the appropriate units.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter15: Additional Aqueous Equilibria
Section: Chapter Questions
Problem 93QRT: When 40.00 mL of a weak monoprotic acid solution is titrated with 0.100-M NaOH, the equivalence...
Related questions
Question
![Just as pH is the negative logarithm of H3O+],
pKa is the negative logarithm of Ka,
pKa = – log Ka
The Henderson-Hasselbalch equation is used to
calculate the pH of buffer solutions:
pH = pK, +log
base]
[acid]
%3D
Notice that the pH of a buffer has a value close to
the pKa of the acid, differing only by the logarithm
of the concentration ratio [base]/[acid].](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F052d6557-f5b4-4f07-a306-e2079f97fc83%2F9ce461b0-a247-4657-a1d0-b4b52c4259b1%2Fvqezd4d_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Just as pH is the negative logarithm of H3O+],
pKa is the negative logarithm of Ka,
pKa = – log Ka
The Henderson-Hasselbalch equation is used to
calculate the pH of buffer solutions:
pH = pK, +log
base]
[acid]
%3D
Notice that the pH of a buffer has a value close to
the pKa of the acid, differing only by the logarithm
of the concentration ratio [base]/[acid].
Transcribed Image Text:Submit
Previous Answers
v Correct
Part B
How many grams of dry NH, Cl need to be added to 1.60 L of a 0.100 mol L- solution of ammonia, NH3 , to prepare
a buffer solution that has a pH of 8.54? K, for ammonia is 1.8 x 10-5.
Express your answer to two significant figures and include the appropriate units.
• View Available Hint(s)
HA
mass of NH4CI =
Value
Units
%3D
Submit
Previous Answers
Incorrect; Try Again; 5 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
