Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.15QAP
Related questions
Question
Transcribed Image Text:Wiew Policies
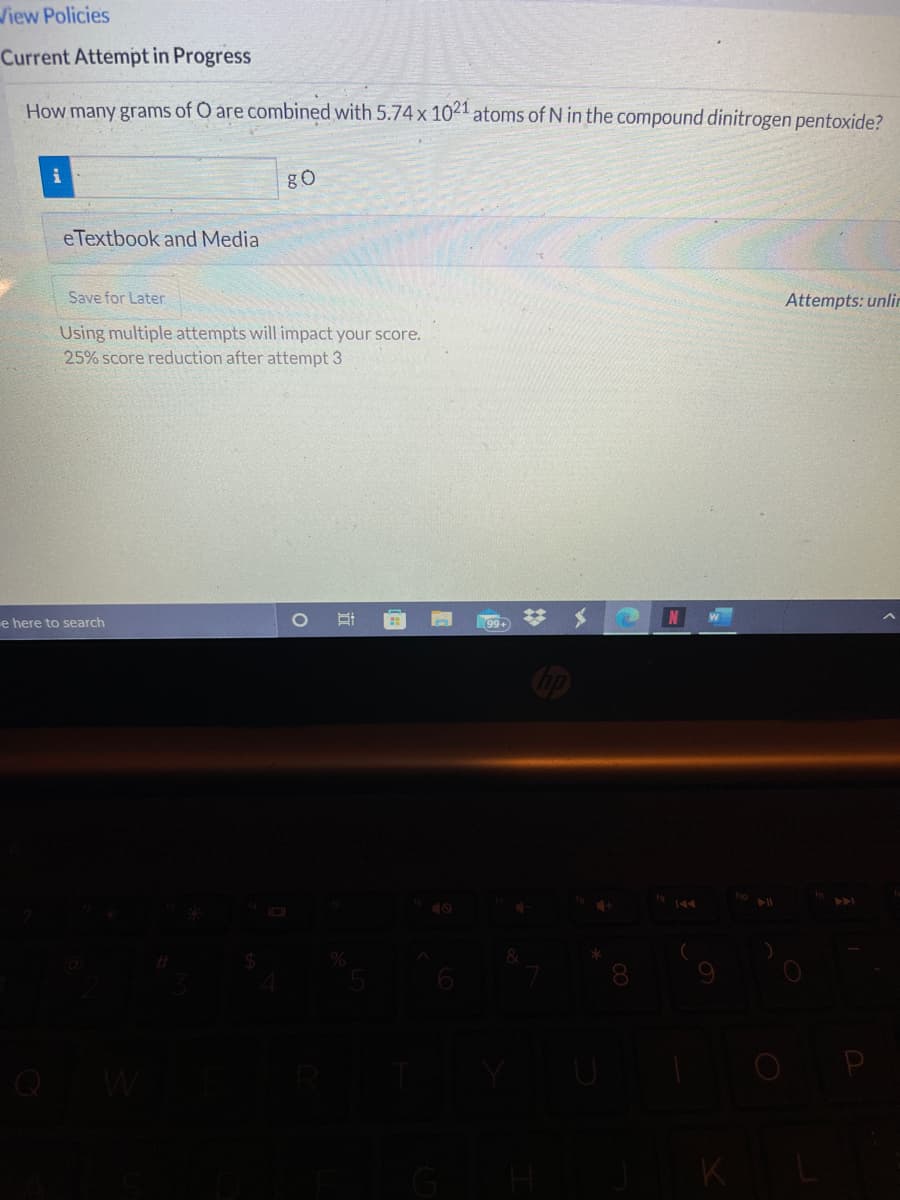
Current Attempt in Progress
How many grams of O are combined with 5.74 x 1021 atoms of N in the compound dinitrogen pentoxide?
eTextbook and Media
Save for Later
Attempts: unlir
Using multiple attempts will impact your score.
25% score reduction after attempt 3
e here to search
99.
144
P
K
Expert Solution
Step 1
First thing is to the Formula of the compound is
N2O5
first use Stiochemitry
1mole of N2O5 will have --->2 x 6.022 x 10^23 atoms
So, 5.74 x 10^21 will have ---> Z moles.
Z Moles = 4.76 x 10^(-3) mol
Compound have Z moles now
1mol of Compound N2O5 have 16 x 5 grams of oxygen.
So Z moles [4.75 x 10^(-3)]of this compound will have
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

