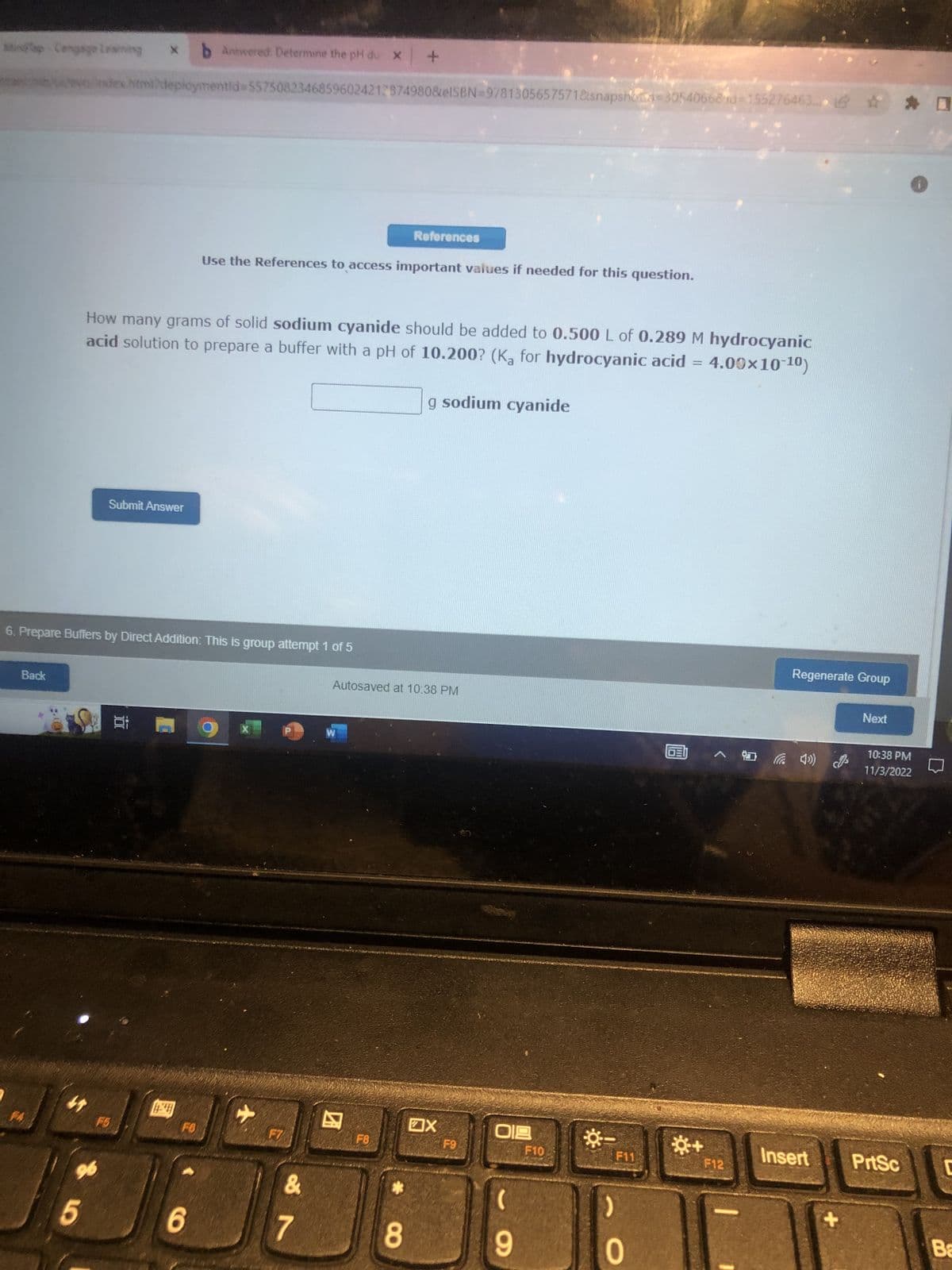
How many grams of solid sodium cyanide should be added to 0.500 L of 0.289 M hydrocyanic acid solution to prepare a buffer with a pH of 10.200? (K₂ for hydrocyanic acid = 4.00×10-10) g sodium cyanide
How many grams of solid sodium cyanide should be added to 0.500 L of 0.289 M hydrocyanic acid solution to prepare a buffer with a pH of 10.200? (K₂ for hydrocyanic acid = 4.00×10-10) g sodium cyanide
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.42QAP
Related questions
Question
Transcribed Image Text:siap Cengage Learning
Back
b Answered. Determine the pH du x +
des Html/deploymentid=557508234685960242128749808eISBN 9781305657571&snapshot-30540668id=155276463... ✰ ✰ 0
5
6. Prepare Buffers by Direct Addition: This is group attempt 1 of 5
96
How many grams of solid sodium cyanide should be added to 0.500 L of 0.289 M hydrocyanic
acid solution to prepare a buffer with a pH of 10.200? (Ka for hydrocyanic acid = 4.00×10-10)
Submit Answer
F5
10:
44
FO
Use the References to access important values if needed for this question.
6
+
F7
&
7
References
W
Autosaved at 10:38 PM
F8
8
g sodium cyanide
X
F9
019
(
9
F10
-☀
()
F11
0
*+
A
F12
Regenerate Group
Insert
+
Next
10:38 PM
11/3/2022
PrtSc
C
Ba
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

