Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.107E
Related questions
Question
Transcribed Image Text:ddp//sduu
3 Honors V...
M Gmail
YouTube
Maps .edu for school
GGrammarly yo O (4) WhatsApp New Tab
school email
Question 11 of 17
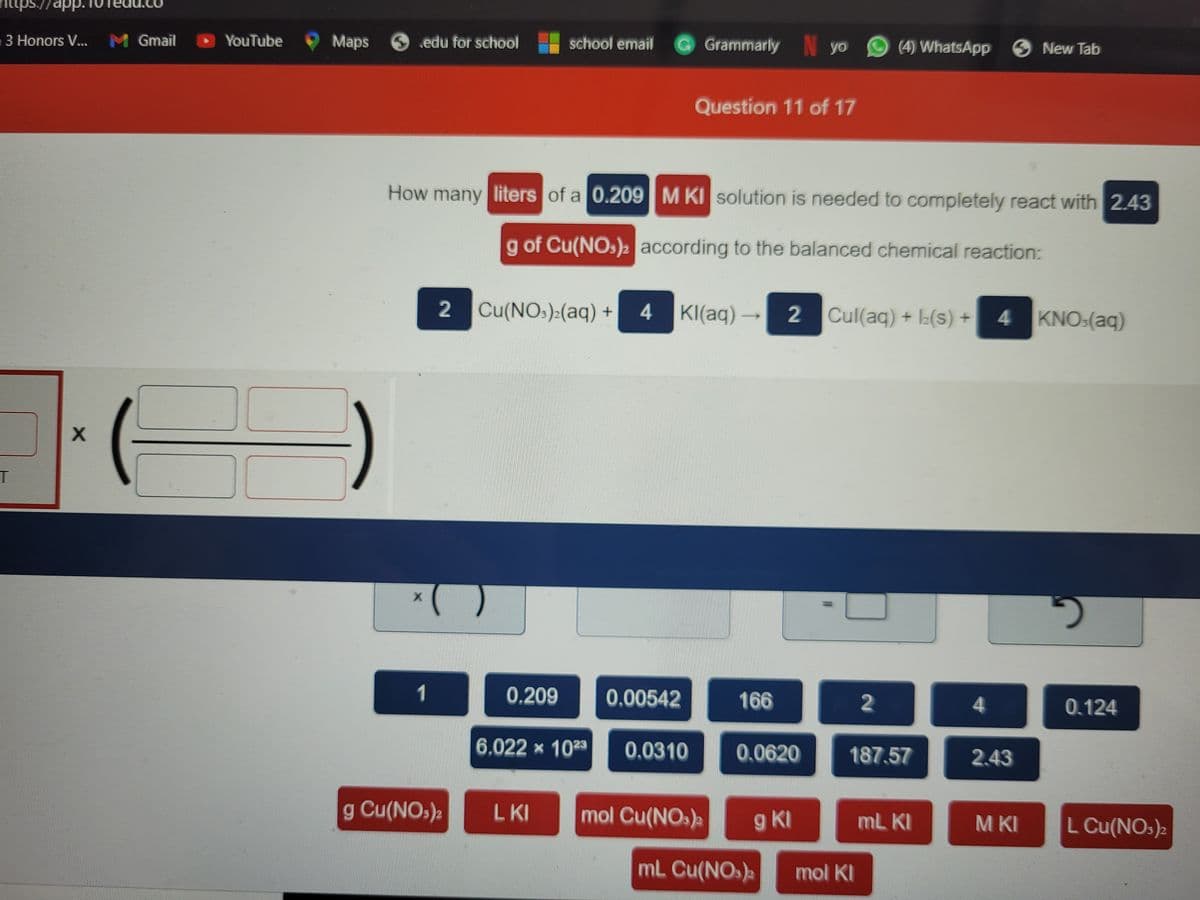
How many liters of a 0.209M KI solution is needed to completely react with 2.43
g of Cu(NOs) according to the balanced chemical reaction:
2 Cu(NO:):(aq) +
4 KI(aq) 2 Cul(aq) + L(s) +
4 KNO:(aq)
1
0.209
0.00542
166
2
4
0.124
6.022 x 1023
0.0310
0.0620
187.57
2.43
g Cu(NO,),
LKI
mol Cu(NO.)a
g KI
mL KI
M KI
L Cu(NOs)2
mL Cu(NOs)
mol KI
Expert Solution
Step 1
Number of moles = mass/molar mass
Molarity = number of moles/volume of solution(in L)
Volume = moles/molarity
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning