Chapter5: Gases
Section: Chapter Questions
Problem 161IP: In the presence of nitric acid, UO2+ undergoes a redox process. It is converted to UO22+ and nitric...
Related questions
Question
100%
Example attached
![Problem #1
An example and checklist is
provided here, also:
Stoichiometry
Volume-Volume Stoichiometry
How many liters of NH3 are needed to
react completely with 30 L NO at STP?
4NH3 (9) + 6NO
(s)
moles
liters
Setup & solve the problem, below:
5N₂ + 6H₂0 (s)
(s)
mass
particles
volume
[at STP]
molar mass
Avogadra's a
molar volume
1.
MOLES
mole ratio
MOLES
molar
Avogadro's a
molar
particles
Which chemical is "desired"?
volume
[at STP]
Convert liters of the given chemical to moles of the given
chemical using the molar volume of a gas at STP.
2. Convert moles of the given chemical to moles of the desired
chemical using mole ratio.
3. Convert moles of the desired chemical to liters of the
desired chemical using the molar volume of a gas at STP.
Answer these questions before you begin:
Which chemical is "given"?](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F789fc5c6-5fa3-4536-a97d-aa6691bce196%2F6bc4521c-b9d3-4ce5-8657-fc540f14f7f8%2F524qet_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Problem #1
An example and checklist is
provided here, also:
Stoichiometry
Volume-Volume Stoichiometry
How many liters of NH3 are needed to
react completely with 30 L NO at STP?
4NH3 (9) + 6NO
(s)
moles
liters
Setup & solve the problem, below:
5N₂ + 6H₂0 (s)
(s)
mass
particles
volume
[at STP]
molar mass
Avogadra's a
molar volume
1.
MOLES
mole ratio
MOLES
molar
Avogadro's a
molar
particles
Which chemical is "desired"?
volume
[at STP]
Convert liters of the given chemical to moles of the given
chemical using the molar volume of a gas at STP.
2. Convert moles of the given chemical to moles of the desired
chemical using mole ratio.
3. Convert moles of the desired chemical to liters of the
desired chemical using the molar volume of a gas at STP.
Answer these questions before you begin:
Which chemical is "given"?
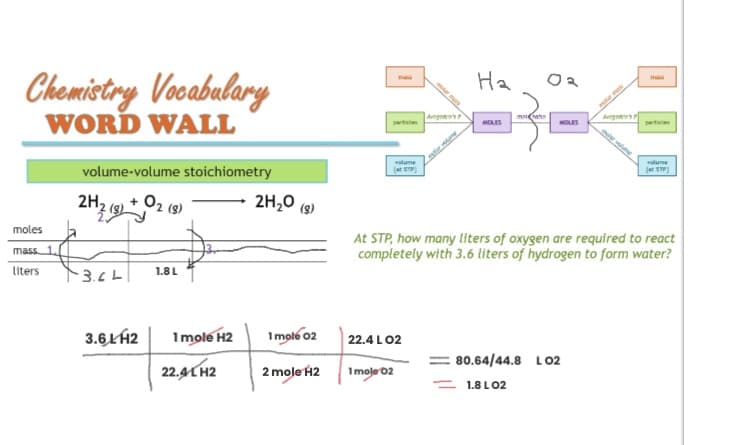
Transcribed Image Text:Chemistry Vocabulary
WORD WALL
moles
mass 1.
liters
volume-volume stoichiometry
2 (3)
2H₂
-3.6L
3.6 LH2
1.8 L
1 mole H2
22.4 LH2
2H₂O (9)
1 mole 02
2 mole H2
volume
(STP)
22.4 L 02
The Ayu
1moje 02
Agora's
На
MOLES
ma
MOLES
At STP, how many liters of oxygen are required to react
completely with 3.6 liters of hydrogen to form water?
partici
80.64/44.8 L02
1.8 LO2
volume
(STP)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning