How many milliliters of water should a chemist addif they want to prepare an 0.200 M aqueous solution with 30.2 g of Naci ? Assume the density of the resulting solution is the same as the water.
How many milliliters of water should a chemist addif they want to prepare an 0.200 M aqueous solution with 30.2 g of Naci ? Assume the density of the resulting solution is the same as the water.
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.106E
Related questions
Question
Im not sure how to fill these in and solve the question
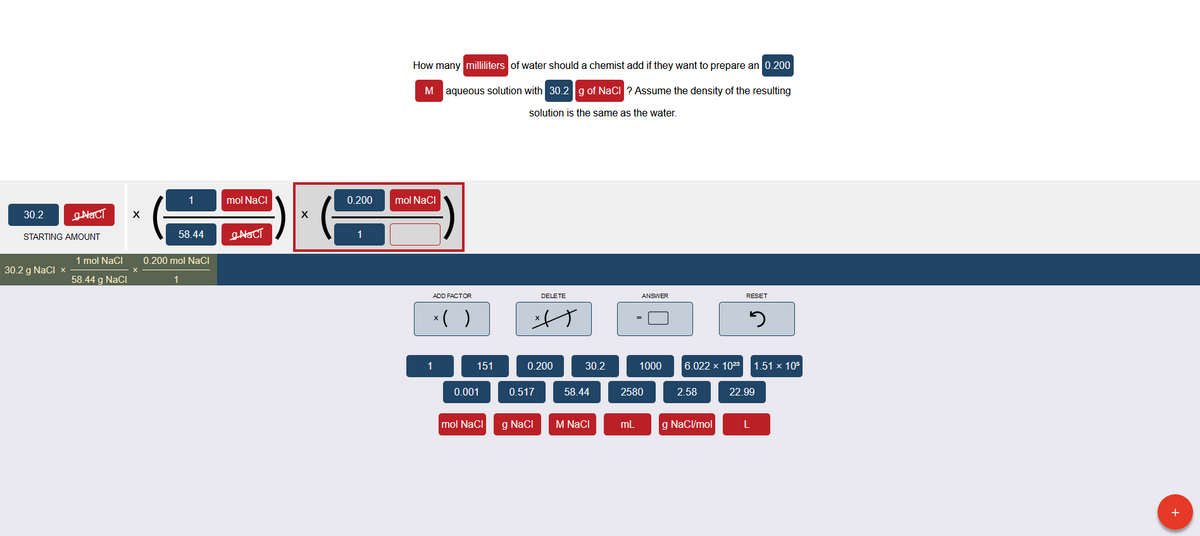
Transcribed Image Text:How many milliliters of water should a chemist add if they want to prepare an 0.200
M aqueous solution with 30.2 g of NaCi ? Assume the density of the resulting
solution is the same as the water.
1
mol NaCl
0.200
mol NaCI
30.2
STARTING AMOUNT
58.44
1
1 mol Naci
0.200 mol NaCI
30.2 g NaCI x
58.44 g NaCI
ADD FACTOR
DELETE
ANSWER
RESET
*( )
1
151
0.200
30.2
1000
6.022 x 10231.51 x 105
0.001
0.517
58.44
2580
2.58
22.99
mol NaCI
g NaCl
M NaCI
mL
g NaC/mol
L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning