how many moles (of molecules or formula units) are in each sample? express your answer to four sig figs 54.69g CF2CL2 26.6 kg Fe(NO3)2 159kgCaO
how many moles (of molecules or formula units) are in each sample? express your answer to four sig figs 54.69g CF2CL2 26.6 kg Fe(NO3)2 159kgCaO
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 90QAP: Calculate the average density of a single Al-27 atom by assuming that it is a sphere with a radius...
Related questions
Question
how many moles (of molecules or formula units) are in each sample?
express your answer to four sig figs
54.69g CF2CL2
26.6 kg Fe(NO3)2
159kgCaO
Transcribed Image Text:I Review I C
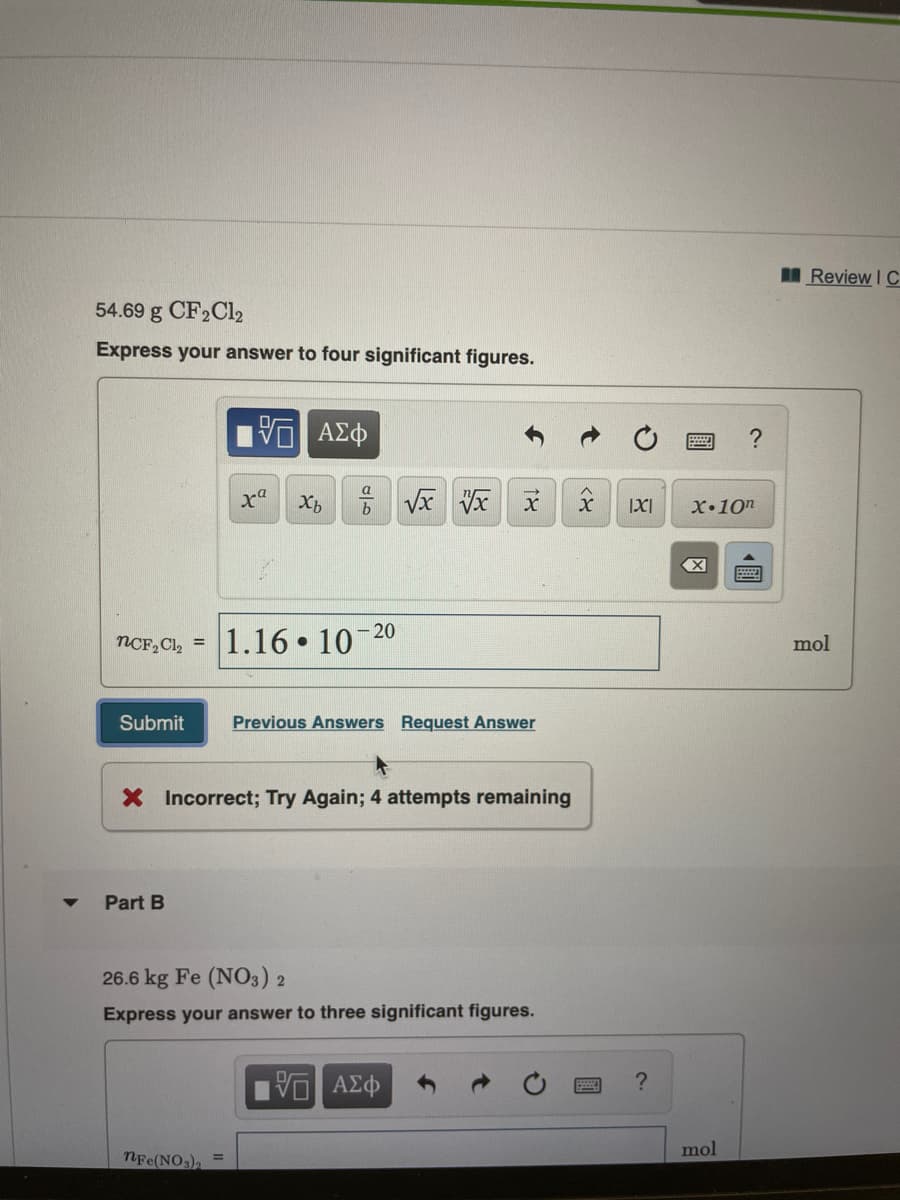
54.69 g CF2C12
Express your answer to four significant figures.
IX|
X•10n
- 20
1.16 10
NCF, Cl, =
mol
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 4 attempts remaining
Part B
26.6 kg Fe (NO3) 2
Express your answer to three significant figures.
να ΑΣφ
mol
NFe(NO)
圓
18
Transcribed Image Text:Submit
Request Answer
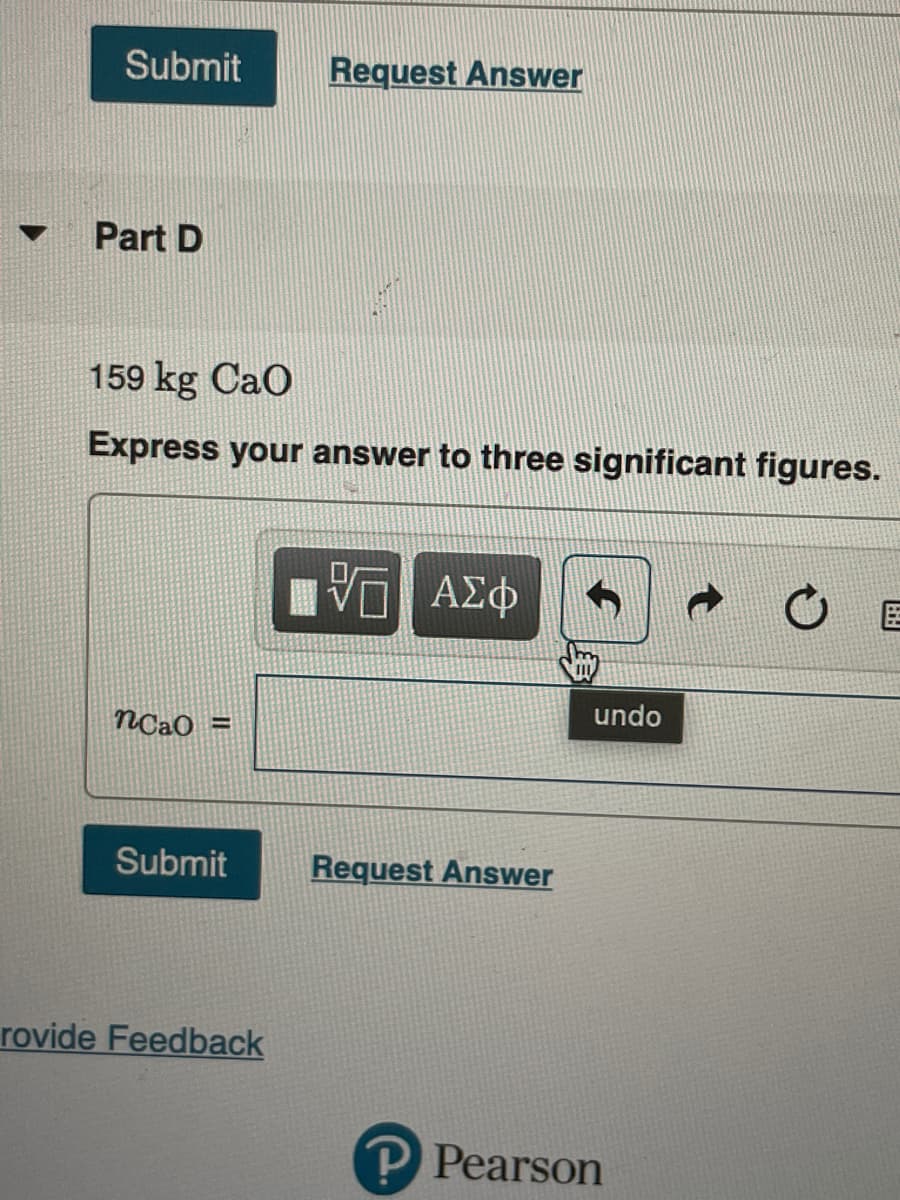
Part D
159 kg Cao
Express your answer to three significant figures.
undo
nCaO
Submit
Request Answer
rovide Feedback
P Pearson
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
