Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter32: Radiochemical Methods
Section: Chapter Questions
Problem 32.4QAP
Related questions
Question

Transcribed Image Text:How many years will it take for 50 microcuries of tritium (Hydrogen-3) to
decay to 3.125 microcuries?
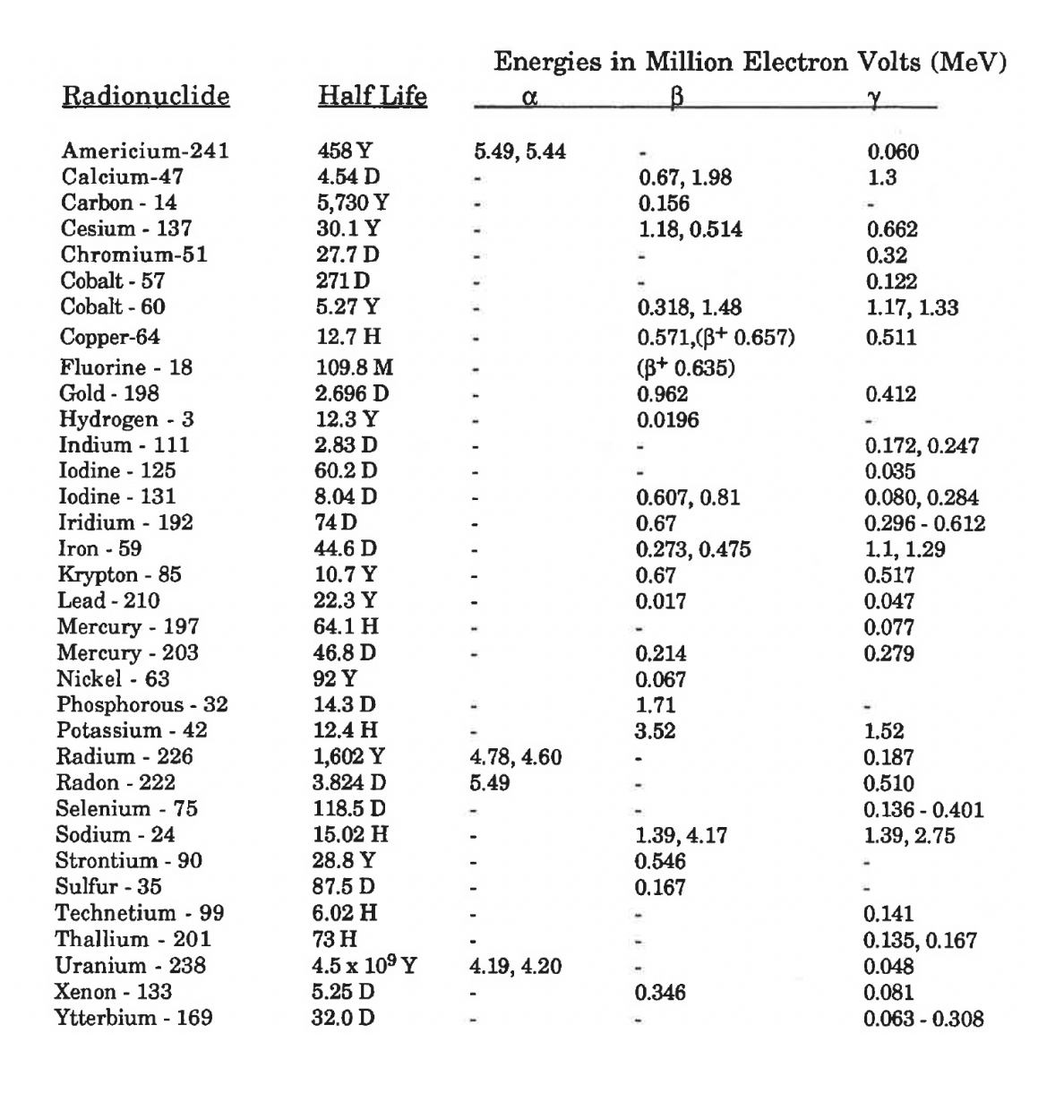
Transcribed Image Text:Energies in Million Electron Volts (MeV)
to
Radionuclide
Half Life
to
Americium-241
458 Y
5.49, 5.44
0.060
1.3
Calcium-47
Carbon - 14
Cesium - 137
0.67, 1.98
0.156
4.54 D
5,730 Y
30.1 Y
27.7 D
271 D
1.18, 0.514
0.662
Chromium-51
0.32
Cobalt - 57
Cobalt - 60
0.122
0.318, 1.48
0.571,(B+ 0.657)
(B+ 0.635)
0.962
5.27 Y
1.17, 1.33
Сopper-64
12.7 H
0.511
Fluorine - 18
Gold - 198
Hydrogen - 3
Indium - 111
Iodine - 125
Iodine - 131
Iridium - 192
Iron - 59
Krypton - 85
Lead - 210
Mercury - 197
Mercury - 203
Nickel - 63
Phosphorous - 32
Potassium - 42
109.8 M
2.696 D
0.412
12.3 Y
0.0196
0.172, 0.247
0.035
0.080, 0.284
0.296 - 0.612
2.83 D
60.2 D
8.04 D
0.607,
0.81
74 D
0.67
0.273, 0.475
0.67
1.1, 1.29
0.517
44.6 D
10.7 Y
22.3 Y
0.017
0.047
64.1 H
0.077
46.8 D
0.214
0.279
92 Y
14.3 D
0.067
1.71
12.4 H
3.52
1.52
Radium - 226
Radon - 222
1,602 Y
3.824 D
4.78, 4.60
5.49
0.187
0.510
0.136 - 0.401
1.39, 2.75
Selenium - 75
118.5 D
Sodium - 24
Strontium - 90
15.02 H
28.8 Y
1.39, 4.17
0.546
0.167
Sulfur - 35
Technetium - 99
Thallium - 201
Uranium - 238
87.5 D
6.02 H
0.141
73 H
4.5 x 109 Y
0.135, 0.167
0.048
0.081
4.19, 4.20
Xenon - 133
5.25 D
0.346
Ytterbium - 169
32.0 D
0.063 - 0.308
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning