How much energy is required to heat 8.6kg of ice from -20℃ to steam at 120C ? The specific heat capacity of ice is 2.097 J/kg C The latent heat of fusion of ice is: Lf = 3.33 × 105J/kg The specific heat capacity of water is 4186J/kg C The latent heat of vaporization of water is: Lv = 22.6 × 105J/kg The specific heat capacity of steam is 1996 J/kg°C DO NOT include units. DO NOT write in scientific notation. Do not include any "spaces" in your answer. 1. The heat energy needed to raise the temperature of ice to 0 degrees Celcius (in Joules) = 2. The heat energy needed to convert ice to water at 0 degrees Celcius (in Joules) = 3. The heat energy needed to raise the temperature of water from 0 degrees to 100 degrees Celcius (in Joules)=
How much energy is required to heat 8.6kg of ice from -20℃ to steam at 120C ? The specific heat capacity of ice is 2.097 J/kg C The latent heat of fusion of ice is: Lf = 3.33 × 105J/kg The specific heat capacity of water is 4186J/kg C The latent heat of vaporization of water is: Lv = 22.6 × 105J/kg The specific heat capacity of steam is 1996 J/kg°C DO NOT include units. DO NOT write in scientific notation. Do not include any "spaces" in your answer. 1. The heat energy needed to raise the temperature of ice to 0 degrees Celcius (in Joules) = 2. The heat energy needed to convert ice to water at 0 degrees Celcius (in Joules) = 3. The heat energy needed to raise the temperature of water from 0 degrees to 100 degrees Celcius (in Joules)=
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Katz, Debora M.
Chapter21: Heat And The First Law Of Thermodynamics
Section: Chapter Questions
Problem 71PQ
Related questions
Question
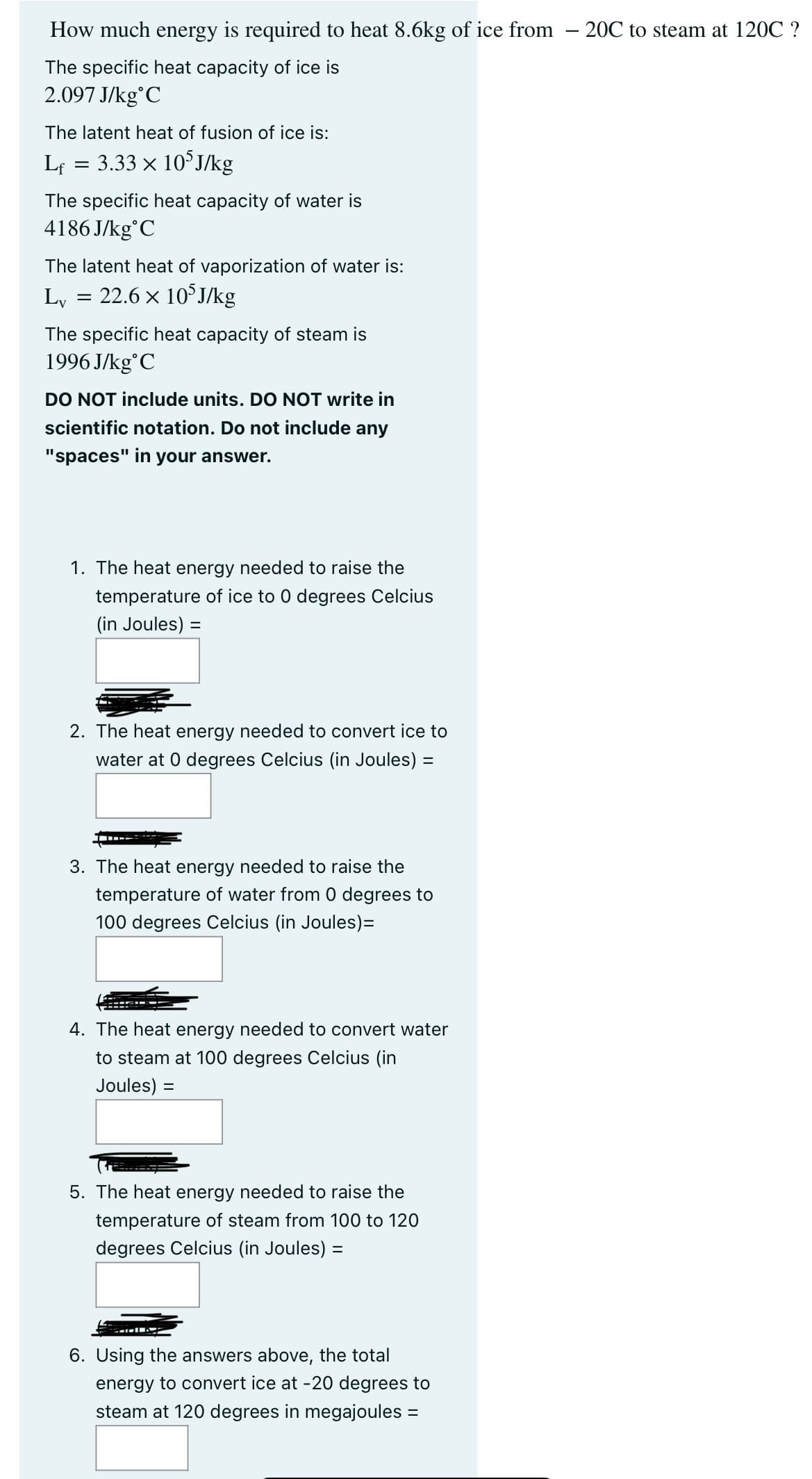
Transcribed Image Text:How much energy is required to heat 8.6kg of ice from -20℃ to steam at 120C ?
The specific heat capacity of ice is
2.097 J/kg C
The latent heat of fusion of ice is:
Lf
=
3.33 × 105J/kg
The specific heat capacity of water is
4186J/kg C
The latent heat of vaporization of water is:
Lv
=
22.6 × 105J/kg
The specific heat capacity of steam is
1996 J/kg C
DO NOT include units. DO NOT write in
scientific notation. Do not include any
"spaces" in your answer.
1. The heat energy needed to raise the
temperature of ice to 0 degrees Celcius
(in Joules) =
2. The heat energy needed to convert ice to
water at 0 degrees Celcius (in Joules) =
3. The heat energy needed to raise the
temperature of water from 0 degrees to
100 degrees Celcius (in Joules)=
4. The heat energy needed to convert water
to steam at 100 degrees Celcius (in
Joules) =
5. The heat energy needed to raise the
temperature of steam from 100 to 120
degrees Celcius (in Joules) =
6. Using the answers above, the total
energy to convert ice at -20 degrees to
steam at 120 degrees in megajoules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning