How much heat? 3kg vapor, 100oC --> 115oC
Q: Sound travels faster in seawater (1522 m/s) than in freshwater (1482 m/s). Why is this? a. Sea...
A: Given: The speed of the sound in seawater is 1522 ms. The speed of the sound in freshwater is 1482 m...
Q: A thermal engine uses up 200 J of heat energy per cycle, while its mechanical work output per cycle ...
A: The thermal engine absorbs 200 J of heat energy per cycle, and the work obtained from this engine pe...
Q: In addition to the normal cooking directions printed on the back of a box of rice, there are also “h...
A: The pressure exerted on the water is maximum at the surface of the world, which is thanks to the l...
Q: An earth satellite moves in a circular orbit with an orbital speed of 6200 m/s. Find (a) the time of...
A: We know that,Orbital speed of satellite, v=GMEr equation 1here, G is the universal gravitatio...
Q: 4. Consider a quantum 3-it initially in the state |v) EC%. Suppose that the 3-it undergoes a reversi...
A:
Q: Two rockets are leaving their space station along perpendicular paths, as measured by a observer on ...
A:
Q: Question #2. Which statement explains how a lunar eclipse happens A. The sun casts a shadow on Earth...
A: Diagram of lunar eclipse :-
Q: A flat circular coil carrying a current of 8.80 A has a magneticdipole moment of 0.194 A . m2 to the...
A: Given: Current=8.80A Magnetic dipole moment =0.194 Am2 Area=4.0 cm2 Formula used: N=Magnetic dipole ...
Q: A block of 2.0 kg oscillates on the end of a spring in SHM with a period of 20 ms [note units]. The...
A: Solution: We know that, Angular speed ω=2πTω=2×3.1420×10-3=314 rad/s From the given expression: ...
Q: Can someone help ASAP?
A: Answer c. Direction C (upwards)
Q: The figure below shows the position of a 20 g oscillating block. If ts on the graph is 4 ms, determi...
A: Time period, ts=4 ms=4×10-3sAmplitude, A=7 cm=0.07 mMass, m=20g=0.02 kgPhase difference, ϕ=π2
Q: 40. What must be the velocity, in meters per beam of electrons if they are to display a de Broglie w...
A: Given: The de Broglie's wavelength is λ=850nm=850×10-9m. Mass of electron is m=9.1×10-31kg.
Q: Charge Q = 5.00 mC is distributed uniformly over the volumeof an insulating sphere that has radius R...
A: U=kq1q2rU=KEkQq(s+R)=12mv2v=2kQqm(s+R)v=2×9×109×5×10-3×3×10-36×10-5(0.08+0.12)=150,000m/s
Q: statics and dynamics
A:
Q: Can someone help asap
A: The capacitance of a parallel plate capacitor is given by, C=Aε0dwhere A is the area of platesd is t...
Q: A 60 kg skydiver jumps from an airplane 4000 m above the Earth. After falling 450 m, he reachesa ter...
A: a) Given, Height (h) = 4000 m Mass (m) = 60 Kg Now, Potential energyinitial = mgh whe...
Q: Belt-zone circulation is not easily visible on Uranus because a. no clouds form in the pure hy...
A: Belt-zone circulation is not easily visible on Uranus because clouds form very deep in the atmospher...
Q: The company where you work has obtained and stored five lasers in a supply room. You have been asked...
A: Given: For Laser A,The power is P=2.6 WDiameter of cylindrical beam,d = 2.6 mmRadius of cylinder bea...
Q: 2. During the peak of the summer period in Sohar, an oil truck loaded 80,000 liters gas. On the way ...
A: Given Initial volume V1 = 80000 litre gas Coefficient of volu...
Q: 1. Find the currents through each resistor given that R1 = 3 N, R2 = 1 N, and R3 = 2 N. The batterie...
A:
Q: In March 2006, two small satellites were discovered orbiting Pluto, one at a distance of 48,000 km a...
A: Given: In March 2006, two small satellites were discovered orbitingPluto, one at a distance of 48,...
Q: 150 g liquid water at 80.0°C is placed in an insulated flask. A 12.0 g ice cube at 0°C is added to t...
A: please see the next step for solution
Q: Answer is also mentioned in picture containing the question. Kindly check the answer before submitti...
A:
Q: Add detail and color
A: The magnetism of the Earth is caused by the dynamo effect. For a circular current-carrying loop, the...
Q: By measurement you determine that soundwaves are spreading out equally in all directions from a poin...
A: Given: The intensity of the sound is I1=0.026Wm2. Distance from source is r1=4.3m.
Q: The below perfectly conductive parallel plate core is divided into three equal parts and a material ...
A:
Q: Consider an electron, a proton, and an alpha particle (a heliumnucleus), each trapped separately in ...
A: i)ground state energyE=n2h28mL2E∝1mso, which particle have lowest mass have higest ground state ener...
Q: Please help asap
A: Concept used: Ampere circuital law can be used to find magnetic field.
Q: Saturn is the most oblate planet in the solar system. Why is Saturn so oblate and what does this tel...
A: Let's understand the oblate shape or structure. An oblate structure is formed by rotating an ellipse...
Q: household contains 8 people, hot water consumption is 60 l/day/person Cold water temperature is 10 °...
A: Given that,Household contains 8 peopleHot water consumption is 60 l/day/personTcold=10°CTHot=58°CHea...
Q: According to the label on a bottle of salad dressing, the volume of the contents is 0.473 liter (L)....
A: Given: To find the volume of the cube is given as,
Q: The cross section for a 2.0-MeV neutron (a typical energy for a neutron released in fi ssion) being a...
A: Given,Cross section σ=0.68 barn=0.68×10-24cm2Thickness of sample t=3.2cmDensity of uranium =19g/cm3
Q: Show transcribed image text a) Refer to Figure #1. A wagon is moving in a straight line with constan...
A: From figure,The force acting in the ball along x-direction is Fxand Force acting along y-direction i...
Q: A block of 2.0 kg oscillates on the end of a spring in SHM with a period of 20 ms [note units]. The...
A: If block one stick with block 2 them mass in SHM will increase. But restoring force and net spring c...
Q: A 12.5 mF capacitor is connected to a power supply that keeps a constant potential difference of 24....
A: Here, we use the formula for energy stored inside a capacitor to get the required values.
Q: Ordinary photographic film reverses black and white, in the sense that the most brightly illuminated...
A: Holography may be a technique which enables a wave front to be recorded and later re-constructed. ...
Q: 2. From the illustration shown in the figure, calculate for the resultant force and the electric fie...
A: Given data; q1=q3=5 μCq2=-2 μCa=0.10 m Now the magnitude of the force exerted by charge q1 and q3 i...
Q: Consider the discrete-time system given below and answer the following questions. y[n] = 0.6x[n] + ...
A: (a). The given discrete-time system is, yn=0.6 xn+0.35 xn-3+0.05 xn-5 Substituting xn=δn where δn=1 ...
Q: What is the volumetric discharge rate in m/s of the tank shown if h = 2.28 m and area of the orifice...
A: Given: The depth of tank is h=2.28 m. The area of orifice is A=0.06 m2. The formula to calculate t...
Q: A square loop (L = 0.20 m) consists of 50 closely wrapped turns, each carrying a current of 0.50 A. ...
A:
Q: You are an alien on an alien planet orbiting the planet's sun in a circular orbit. You want to find ...
A: We know that,Time period of planet around the sun is, T=2πr3GM equation 1Given that, T=1.21×107...
Q: For the system shown, the coefficient of kinetic friction between the surface and the 2 kg block is ...
A: The free-body diagram of the 1 kg mass is given below;
Q: Please help asap
A: When does the light bulb glow brightest. given: Circuit:
Q: A coaxial cable has inner conductor radius a=7.6cm and outer conductor radius b=24.6cm. The medium b...
A: given a = 7.6 cm = 0.076 mb = 24.6 cm =0.246 mρl = 16.2 nc/m = 16.2 x 10-9 c/m
Q: Calculate the de Broglie wavelength of (a) a tennis ball of mass 57 g traveling 25 m/s (about 56 mph...
A: Here, we use the formula of de Broglie wavelength to get the values for given systems.
Q: NEWTON’S RING Experiment I want Analysis for this graph
A: The slope of this graph will be (12.4609 - 6.4516)/(5 - 1) = 1.502 Which is okay .The problem wit...
Q: Raindrops hitting the side windows of a car in motion often leave diagonal streaks even if there is ...
A: Given Raindrops hitting the side windows of a car in motion often leave diagonal streaks even if the...
Q: Blood of density 1,060 kg/m3 and viscosity 0.0034 Pa/s flows through an aorta with radius 0.013 m. ...
A: Let us denote the Womersley number with α. Here we have density of fluid (ρ) , viscosity of fluid (...
Q: (3) An object of mass 10 kg is dropped from a flying plane. The force of air resistance is 0.07v, vh...
A: How long has the object travelled before it is within 10% of its terminal velocity. given, mass of o...
Q: Answer and solve the question below.
A: Given data F2=100 F1
How much heat? 3kg vapor, 100oC --> 115oC
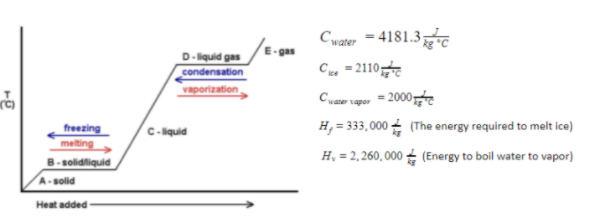
Step by step
Solved in 2 steps
