How much heat (in kJ) must be added to completely transform the liquid water (at its boiling point) to water vapor, still at its boiling point? О 335 O 670 O 2,260 O 4,520 О -з35 O -670 O -2,260 O -4,520 O None of the above.
How much heat (in kJ) must be added to completely transform the liquid water (at its boiling point) to water vapor, still at its boiling point? О 335 O 670 O 2,260 O 4,520 О -з35 O -670 O -2,260 O -4,520 O None of the above.
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 102AP: In 1701, the Danish astronomer Ole Rømer proposed a temperature scale with two fixed points,...
Related questions
Question
Consider a block of ice, with a mass of 2.00 kg and an initial temperature of -8.00°C.
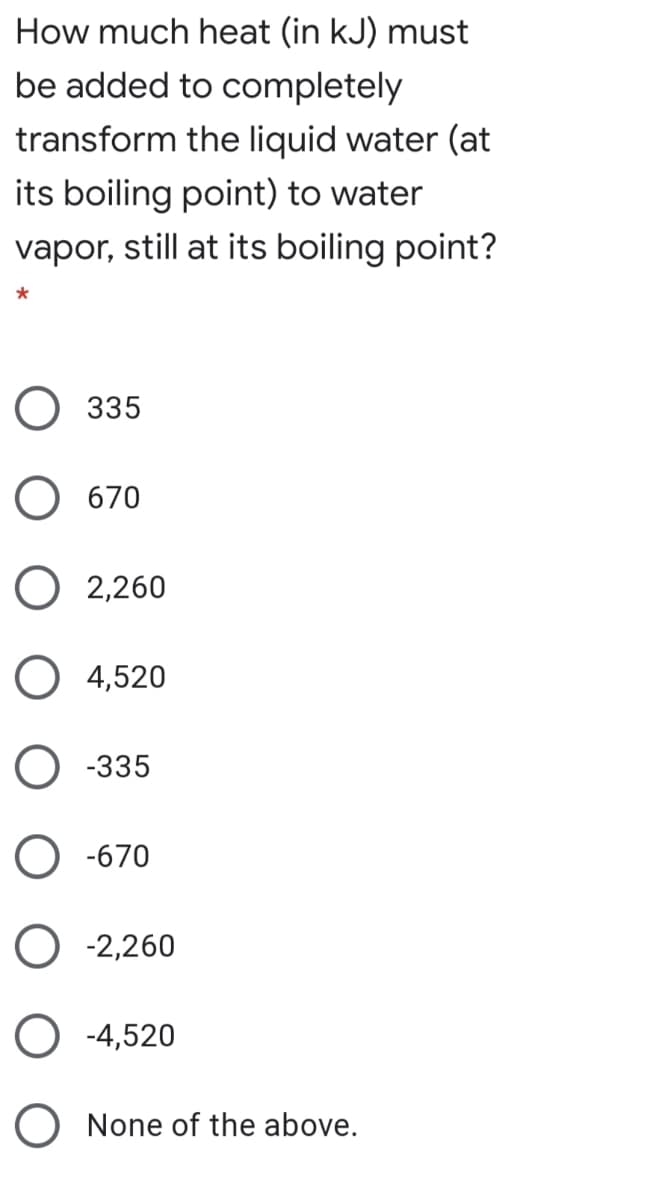
Transcribed Image Text:How much heat (in kJ) must
be added to completely
transform the liquid water (at
its boiling point) to water
vapor, still at its boiling point?
О 335
O 670
O 2,260
O 4,520
О з35
O -670
O -2,260
O -4,520
O None of the above.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning