How was 146 degrees Celcius obtained? I know how the 5250 mmhg was obtained.
How was 146 degrees Celcius obtained? I know how the 5250 mmhg was obtained.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
How was 146 degrees Celcius obtained? I know how the 5250 mmhg was obtained.
Transcribed Image Text:||!
PDF *Elementary Principles of Chemic X +
69°F
Mostly cloudy
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDFDrive%20).pdf
Draw
T Read aloud
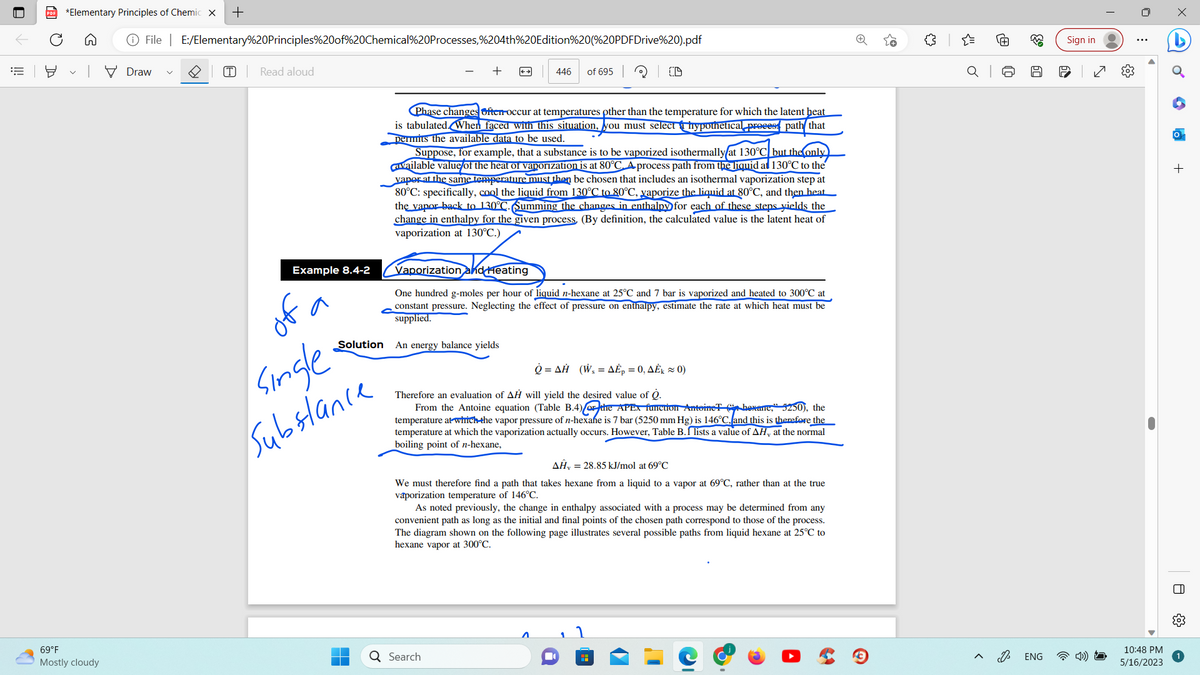
Example 8.4-2
Single
Substance
+
446 of 695
Phase changes ofter-occur at temperatures other than the temperature for which the latent heat
is tabulated. When faced with this situation, you must select hypothetical process path that
permits the available data to be used.
Solution An energy balance yields
Suppose, for example, that a substance is to be vaporized isothermally at 130°C but the only
available value of the heat of vaporization is at 80°C. A process path from the liquid al 130°C to the
yaper at the same temperature must then be chosen that includes an isothermal vaporization step at
80°C: specifically, cool the liquid from 130°C to 80°C, vaporize the liquid at 80°C, and then heat
the vapor back to 130°C. Summing the changes in enthalpy for each of these steps yields the
change in enthalpy for the given process. (By definition, the calculated value is the latent heat of
vaporization at 130°C.)
Vaporization and Heating
One hundred g-moles per hour of liquid n-hexane at 25°C and 7 bar is vaporized and heated to 300°C at
constant pressure. Neglecting the effect of pressure on enthalpy, estimate the rate at which heat must be
supplied.
ETA
Q Search
ῥ =ΔΗ (W, = ΔÉp = 0, ΔΕk = 0)
Therefore an evaluation of AH will yield the desired value of Q.
From the Antoine equation (Table B.4) the APEX function Antoine hexame, 5250), the
temperature at which the vapor pressure of n-hexafe is 7 bar (5250 mm Hg) is 146°C, and this is therefore the
temperature at which the vaporization actually occurs. However, Table B. lists a value of AH, at the normal
boiling point of n-hexane,
AH, = 28.85 kJ/mol at 69°C
We must therefore find a path that takes hexane from a liquid to a vapor at 69°C, rather than at the true
vaporization temperature of 146°C.
As noted previously, the change in enthalpy associated with a process may be determined from any
convenient path as long as the initial and final points of the chosen path correspond to those of the process.
The diagram shown on the following page illustrates several possible paths from liquid hexane at 25°C to
hexane vapor at 300°C.
■
H
{"
Ⓡ
J
63
60
ENG
Sign in
(0)
10:48 PM
5/16/2023
+
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The