Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.21QAP
Related questions
Question
Question: how would you expect solubility of product of potassium bitartrate to vary between distilled water and a KNO3 solution?
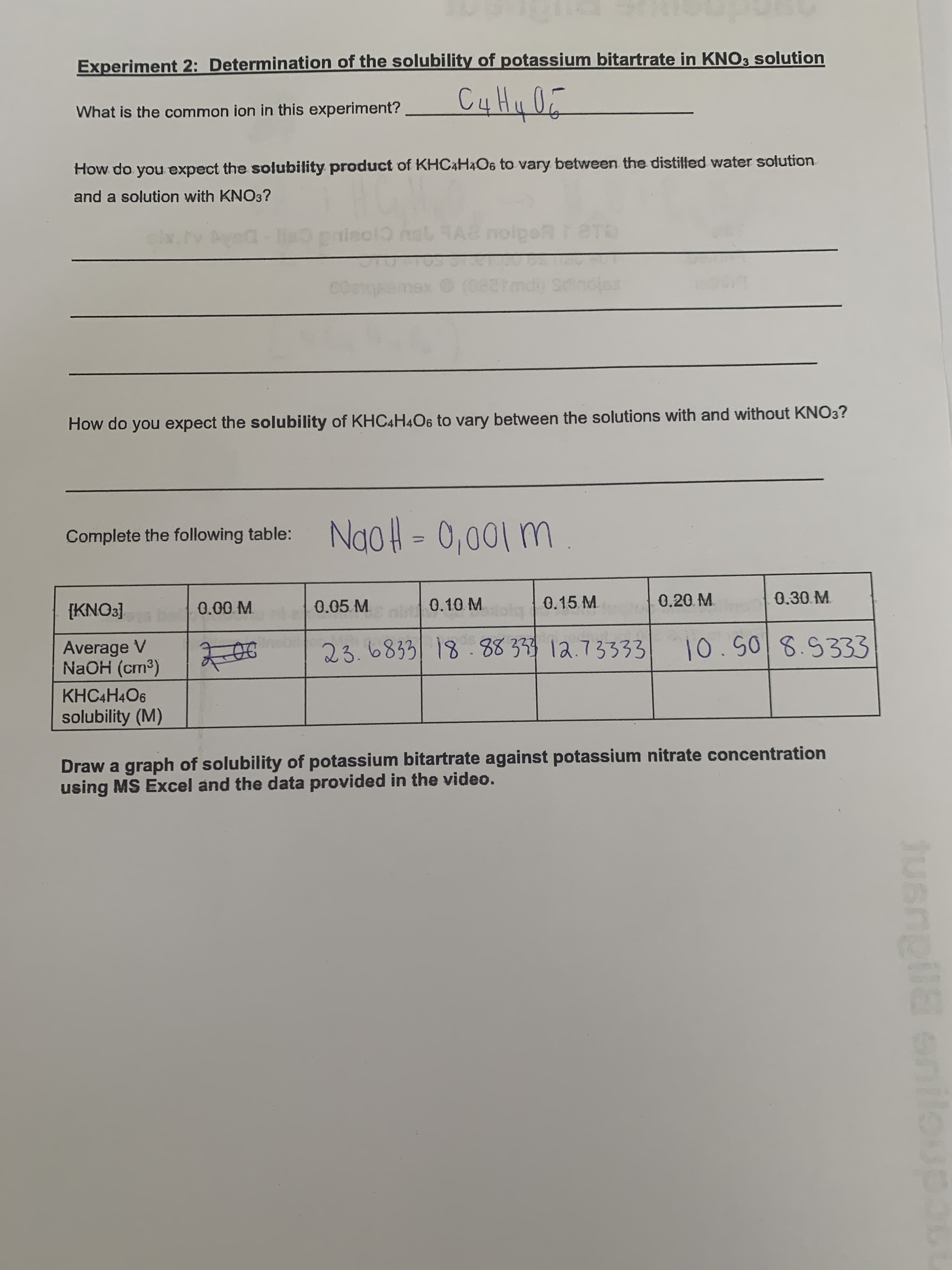
Transcribed Image Text:Experiment 2: Determination of the solubility of potassium bitartrate in KNO3 solution
What is the common ion in this experiment?
ор мон
and a solution with KNO3?
How do you expect the solubility product of KHC4H4O6 to vary between the distilled water solution
How do you expect the solubility of KHC4H4O6 to vary between the solutions with and without KNO3?
Complete the following table: NaoH = 0,001 m
0.05.M.
0.10 M.
0.15 M.
0.20. M
0.30.M.
600 M.
Average V
NaOH (cm³)
23.6833| 18.88333 12.73333
10.508.5333
KHC4H4O6
solubility (M)
Draw a graph of solubility of potassium bitartrate against potassium nitrate concentration
using MS Excel and the data provided in the video.

Transcribed Image Text:Experiment 2
Experiment 2: Determination of the solubility of potassium bitartrate in KN03 solution
Apparatus and equipment
Chemicals
Analytical balance
Potassium bitartrate ( KHC4H406)
250 mL beaker (2)
Distilled water
Filter paper (dry)
Standard solution 2: NaOHaq (0.01 M)
Funnel
Indicator: phenolphthalein
Burette
5 x different KNO3 solutions
Pipette
(0.05 M, 0.10 M, 0.15 M 0.20 M, 0.30 M)
Thermometer (at tutor)
Ehrlenmeyer flasks (4)
Experimental procedure
6. Repeat the above experiment (1 8) replacing the deionized water in the solution by 150 mL of the assigned potassium nitrate (KNO3) solution, using sodium
hydroxide solution (standard 2) for titration.
7. Calculate the solubility and solubility product of potassium bitartrate in both water and the potassium nitrate solution. Show all calculations.
8. Use the collected results to plot a graph of the solubility of potassium bitartrate against potassium nitrate concentration. Include the solubility of potassium bitartrate
in pure water (0.0 M potassium nitrate) on the graph.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT