I An early model of the atom, proposed by Rutherford after his discovery of the atomic nucleus, had a positive point charge +Ze (the nucleus) at the center of a sphere of radius R with uniformly distributed negative charge -Ze. Z is the atomic number, the number of protons in the nucleus and the number of electrons in the negative sphere. a. Show that the electric field inside this atom is Ze 1 Ein 4πο R b. What is E at the surface of the atom? Is this the expected value? Explain. c. A uranium atom has Z= 92 and R = 0.10 nm. What is the electric field strength at r =R?
I An early model of the atom, proposed by Rutherford after his discovery of the atomic nucleus, had a positive point charge +Ze (the nucleus) at the center of a sphere of radius R with uniformly distributed negative charge -Ze. Z is the atomic number, the number of protons in the nucleus and the number of electrons in the negative sphere. a. Show that the electric field inside this atom is Ze 1 Ein 4πο R b. What is E at the surface of the atom? Is this the expected value? Explain. c. A uranium atom has Z= 92 and R = 0.10 nm. What is the electric field strength at r =R?
Related questions
Question
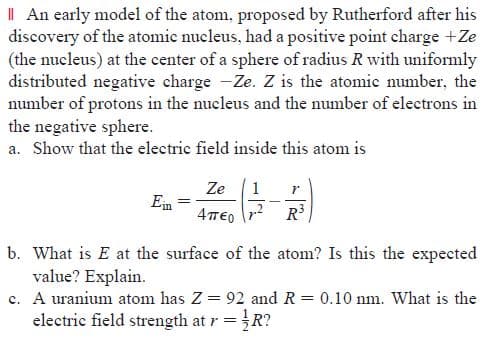
Transcribed Image Text:I| An early model of the atom, proposed by Rutherford after his
discovery of the atomic nucleus, had a positive point charge +Ze
(the nucleus) at the center of a sphere of radius R with uniformly
distributed negative charge -Ze. Z is the atomic number, the
number of protons in the nucleus and the number of electrons in
the negative sphere.
a. Show that the electric field inside this atom is
Ze
1
Ein
4πο
R3
b. What is E at the surface of the atom? Is this the expected
value? Explain.
c. A uranium atom has Z = 92 and R = 0.10 nm. What is the
electric field strength at r =R?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images
