I.A Study of Intermolecular Forces of Attraction Professor Rowan is preparing for his experiment in Sinnoh Lab. The structures of the substances that he will use are presented below: Substance Turtwig Substance Piplup Substance Chimchar H H %3D %3D H H H. Molar Mass: 106.12g/mol Molar mass: 122.12g/mol Molar mass: 92.14g/mol A. What is/are the IMFA present in each of the substances? (LDF - London Dispersion Forces, DDF - Dipole-dipole Forces, HB- Hydrogen Bonding) B. Determine the most possible melting point of each of the substances. (Possible melting points: 122 °C, -26 °C, -95 °C) C. Rowan's Apprentice, Dawn, needs a solid starting material in her experiment at room temperature (at 25°C). Among the three substances, what is Dawn's starting material?
I.A Study of Intermolecular Forces of Attraction Professor Rowan is preparing for his experiment in Sinnoh Lab. The structures of the substances that he will use are presented below: Substance Turtwig Substance Piplup Substance Chimchar H H %3D %3D H H H. Molar Mass: 106.12g/mol Molar mass: 122.12g/mol Molar mass: 92.14g/mol A. What is/are the IMFA present in each of the substances? (LDF - London Dispersion Forces, DDF - Dipole-dipole Forces, HB- Hydrogen Bonding) B. Determine the most possible melting point of each of the substances. (Possible melting points: 122 °C, -26 °C, -95 °C) C. Rowan's Apprentice, Dawn, needs a solid starting material in her experiment at room temperature (at 25°C). Among the three substances, what is Dawn's starting material?
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter5: Distillation
Section: Chapter Questions
Problem 4Q
Related questions
Question
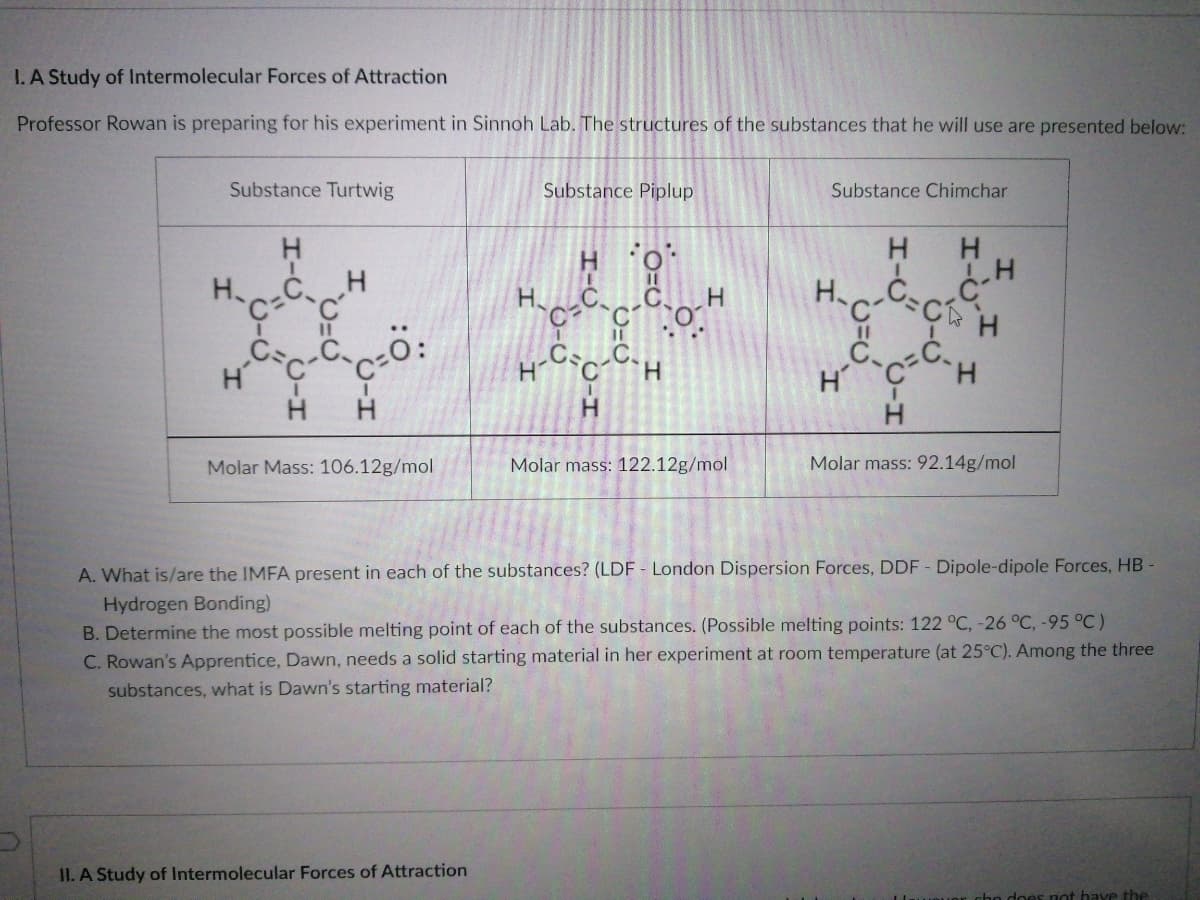
Transcribed Image Text:I. A Study of Intermolecular Forces of Attraction
Professor Rowan is preparing for his experiment in Sinnoh Lab. The structures of the substances that he will use are presented below:
Substance Turtwig
Substance Piplup
Substance Chimchar
H
H CH
H
C-H
%3D
H
H.
H
3.
H.
H.
Molar Mass: 106.12g/mol
Molar mass: 122.12g/mol
Molar mass: 92.14g/mol
A. What is/are the IMFA present in each of the substances? (LDF - London Dispersion Forces, DDF - Dipole-dipole Forces, HB -
Hydrogen Bonding)
B. Determine the most possible melting point of each of the substances. (Possible melting points: 122 °C, -26 °C, -95 °C)
C. Rowan's Apprentice, Dawn, needs a solid starting material in her experiment at room temperature (at 25°C). Among the three
substances, what is Dawn's starting material?
II. A Study of Intermolecular Forces of Attraction
s not have the
I-U
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole