Ibuprofen is a weak acid with a pK, of 4.9 (structure is shown with the ionizable hydrogen marked with a star). Ibuprofen is absorbed through the stomach and the small intestine. In these tissues, absorption is a function of polarity. Charged and very polar molecules are absorbed slowly; neutral hydrophobic molecules absorb quickly. If the stomach pH is about 1.5 and the small intestine pH is about 6, whem (and why) will more ibuprofen be absorbed into the bloodstream? OH* HgC HC CH ECH H3C „CH2 CH ČH3 More ibuprofen will be absorbed in the small intestine because it will be uncharged due to the pH being greater than the pK. O More ibuprofen will be absorbed in the stomach because it will be charged due to the pH being lower than the pK. O More ibuprofen will be absorbed in the small intestine because it will be charged due to the pH being greater than the pK. More ibuprofen will be absorbed in the stomach because it will be uncharged due to the pH being lower than the pk. Ibuprofen will be absorbed equally well in both the stomach and small intestine.
Ibuprofen is a weak acid with a pK, of 4.9 (structure is shown with the ionizable hydrogen marked with a star). Ibuprofen is absorbed through the stomach and the small intestine. In these tissues, absorption is a function of polarity. Charged and very polar molecules are absorbed slowly; neutral hydrophobic molecules absorb quickly. If the stomach pH is about 1.5 and the small intestine pH is about 6, whem (and why) will more ibuprofen be absorbed into the bloodstream? OH* HgC HC CH ECH H3C „CH2 CH ČH3 More ibuprofen will be absorbed in the small intestine because it will be uncharged due to the pH being greater than the pK. O More ibuprofen will be absorbed in the stomach because it will be charged due to the pH being lower than the pK. O More ibuprofen will be absorbed in the small intestine because it will be charged due to the pH being greater than the pK. More ibuprofen will be absorbed in the stomach because it will be uncharged due to the pH being lower than the pk. Ibuprofen will be absorbed equally well in both the stomach and small intestine.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 40QRT: Leucine is an amino acid with this Lewis structure:
Write the Lewis structure for the zwitterion...
Related questions
Question
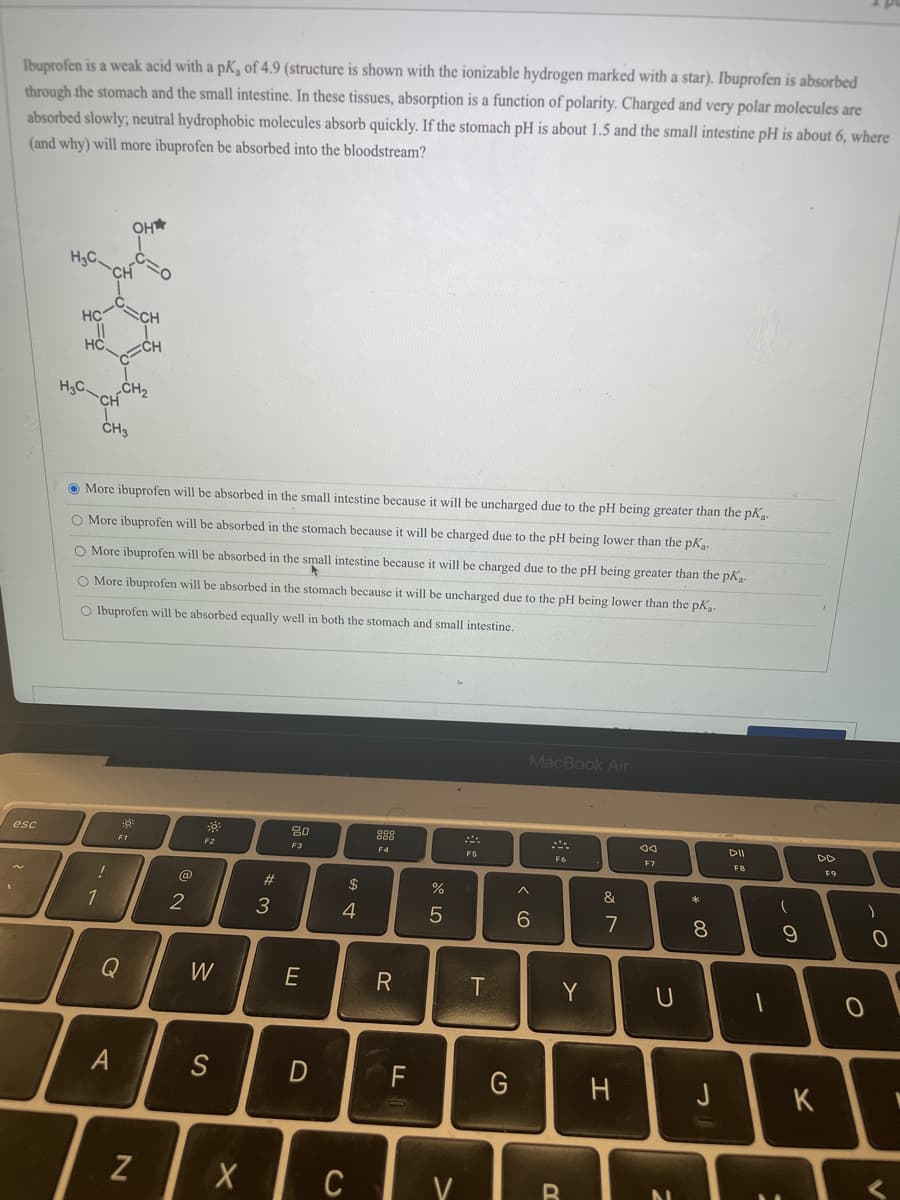
Transcribed Image Text:Ibuprofen is a weak acid with a pK, of 4.9 (structure is shown with the ionizable hydrogen marked with a star). Ibuprofen is absorbed
through the stomach and the small intestine. In these tissues, absorption is a function of polarity. Charged and very polar molecules are
absorbed slowly; neutral hydrophobic molecules absorb quickly. If the stomach pH is about 1.5 and the small intestine pH is about 6, where
(and why) will more ibuprofen be absorbed into the bloodstream?
OH
HC
H3C CH2
CH3
O More ibuprofen will be absorbed in the small intestine because it will be uncharged due to the pH being greater than the pK.
O More ibuprofen will be absorbed in the stomach because it will be charged due to the pH being lower than the pK.
O More ibuprofen will be absorbed in the small intestine because it will be charged due to the pH being greater than the pk
O More ibuprofen will be absorbed in the stomach because it will be uncharged due to the pH being lower than the pK.
O Ibuprofen will be absorbed equally well in both the stomach and small intestine.
MacBook Air
80
888
esc
DII
DD
F1
F2
F3
F4
F5
F7
FB
F9
@
#3
$
&
1
3
4
6
8.
Q
W
T
Y
A
S
D
F
K
Z
C
この
* LO
Expert Solution
Step 1
The correct option is:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning