If a chemical bond vibrates at a frequency w, the average energy of the bond turns out to be equal to the following complicated expression: hw, E, nhwe=nhwß (E) + (7) (Where 3 = kgT, not that it will matter much here.) Take the above statement as true (because it is) and prove or disprove the following assertion: Σ d (E) ; In `e¯nhwB dB (8) 2
If a chemical bond vibrates at a frequency w, the average energy of the bond turns out to be equal to the following complicated expression: hw, E, nhwe=nhwß (E) + (7) (Where 3 = kgT, not that it will matter much here.) Take the above statement as true (because it is) and prove or disprove the following assertion: Σ d (E) ; In `e¯nhwB dB (8) 2
Algebra & Trigonometry with Analytic Geometry
13th Edition
ISBN:9781133382119
Author:Swokowski
Publisher:Swokowski
Chapter11: Topics From Analytic Geometry
Section11.4: Plane Curves And Parametric Equations
Problem 44E
Related questions
Question
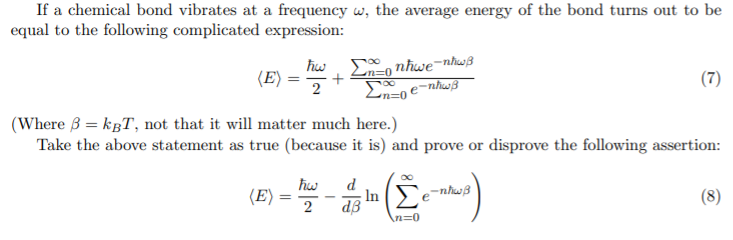
Transcribed Image Text:If a chemical bond vibrates at a frequency w, the average energy of the bond turns out to be
equal to the following complicated expression:
hw, E, nhwe=nhwß
(E)
+
(7)
(Where 3 = kgT, not that it will matter much here.)
Take the above statement as true (because it is) and prove or disprove the following assertion:
Σ
d
(E)
; In `e¯nhwB
dB
(8)
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Linear Algebra: A Modern Introduction
Algebra
ISBN:
9781285463247
Author:
David Poole
Publisher:
Cengage Learning

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Linear Algebra: A Modern Introduction
Algebra
ISBN:
9781285463247
Author:
David Poole
Publisher:
Cengage Learning