If a machine does 75.44 kJ of work after an input of 97.31 kJ of heat, what is the change in internal energy for the machine? Round your answer to 2 decimal places.
If a machine does 75.44 kJ of work after an input of 97.31 kJ of heat, what is the change in internal energy for the machine? Round your answer to 2 decimal places.
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
Section: Chapter Questions
Problem 6RE
Related questions
Question
use the second image as reference for standards
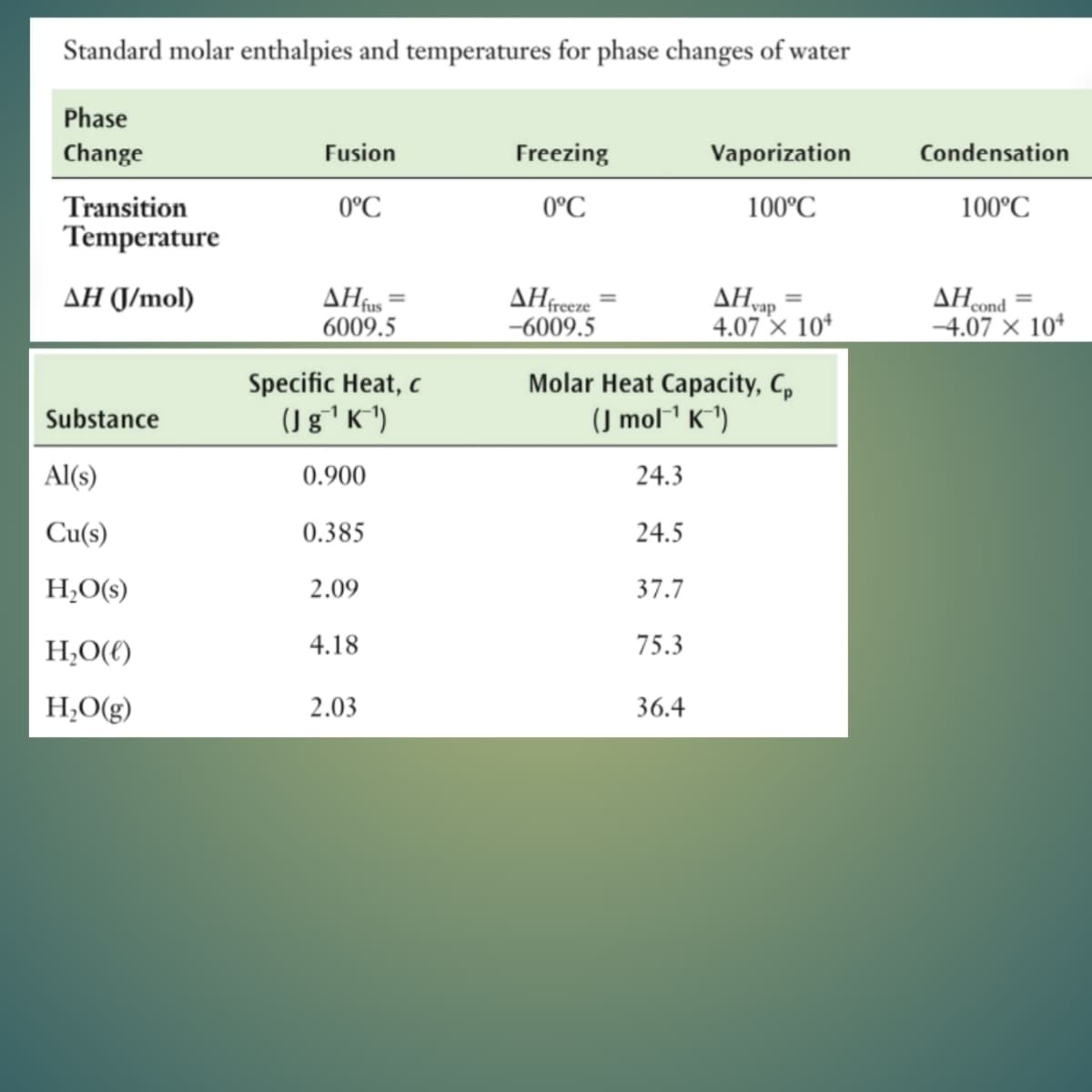
Transcribed Image Text:Standard molar enthalpies and temperatures for phase changes of water
Phase
Change
Fusion
Freezing
Vaporization
Condensation
Transition
0°C
0°C
100°C
100°C
Temperature
ΔΗ
6009.5
AHvap
4.07 × 10*
AHcond
-4.07 × 10*
AH (J/mol)
AHfreeze
-6009.5
Specific Heat, c
(Jg'K*)
Molar Heat Capacity, C,
(J mol1 K^1)
Substance
Al(s)
0.900
24.3
Cu(s)
0.385
24.5
H¿O(s)
2.09
37.7
H,O(()
4.18
75.3
H;O(g)
2.03
36.4

Transcribed Image Text:If a machine does 75.44 kJ of work after an input of 97.31 kJ of heat, what is the change in internal energy for the machine?
Round your answer to 2 decimal places.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning