If Co/Co³+, Co²+/Co³+, Fe / Fe³+, Fe²+/ Fe³+, std. oxidation potential are,-0.416 v,-1.18 v, 0.0367 v, -0.77 v, respectively, then the std. potential of the cell Fe/Fe²+ // Co2+ / Co,
Q: 0.00089 moles of thiamine hydrochloride reacted with .5ml of water , then 0.000065 moles of ethanol…
A: Benzoin condensation is the condensation of two molecules of benzaldehyde to give α-hydroxy ketone…
Q: Question 2 of 4 O Macmillan Learning > Organic Chemistry Loudon | Parise SEVENTH EDITION Label each…
A: It is based on the concept of acid and Base Here we are required to identify the compound as Lewis…
Q: 1. What is the composition of the nuclei modeled by these symbols? How many protons and neutrons?…
A: Two questions based on nuclear reactions. Explanation for nuclear notation and the conversion into…
Q: 15.20 Give structural formulas for the intermediates and products indicated by letters in the…
A: The above question is based on organic reaction and mechanism. We have to discuss about the product…
Q: Zinc sulfide reacts with oxygen according to the reaction 2ZnS(s)+3O2(g)→2ZnO(s)+2SO2(g) A…
A: 2ZnS(s) + 3O2(g)→2ZnO(s) + 2SO2(g) Initially, moles of ZnS = 3.0 mol moles of O2 = 5.6 mol Amount of…
Q: 10. Given the following decomposition reaction, calculate the moles of lithium chloride produced…
A: Given,The balanced decomposition reaction:2 LiClO3(s) →∆ 2 LiCl(s) + O2(g)moles of LiClO3 = 5.00…
Q: a) Show using free radical reaction how you can prepare benzyl bromide from toluene.…
A: Given :- a) toluene ——> benzyl bromide b) benzyl bromide ———> phenylmethanamine To show :-…
Q: Consider the three structures. 1,1-dimethylcyclobutane IIII... (a) Which of these structures has the…
A: The angle strain arises due to deviation from the bond angle for a particular type of hybridization.…
Q: Vanadium atoms absorb light at 650 nm. If g* is 3 and g, is 2 calculate the relative population of…
A: In the given question we have to calculate the relative population of the excited state, Relative…
Q: Which mechanism do the following nucleophiles/bases favor? хон OH SH Drag answer here Drag ans ver…
A: Tertiary alcohols being bulky enough favour both E1 & SN1 by virtue of their stable tertiary…
Q: At -5.21 °C the concentration equilibrium constant K = 2.9 × 105 for a certain reaction. с Here are…
A: For exothermic reaction, equilibrium constant value decreases with increase of temperature For…
Q: a) Assign oxidation numbers to the atoms in the following compounds or ions: b) Cr₂O7²- IF7 Atom Cr…
A: Oxidation: It involves Loss of electrons. Reduction: It involves gain of electrons.
Q: At-14.4 °C the concentration equilibrium constant K, -9.3x10 for a certain reaction. Here are some…
A: If energy is released, reaction exothermicIf energy is absorbed, reaction is endothermicFor…
Q: -3 2 AsO4³ +3 Ca²+ ---> Ca3(AsO4)2(S) Your goal here is to determine what is left over when we mix a…
A: To determine what is left over when mixing the given solutions, we need to compare the moles of the…
Q: 1. Which is correct: a) The neutral EDTA is triprotic b) EDTA (Y-4) is tetradentate c) The blue EBT…
A: In first question, there are four statements given in which we have to figure out which is correct…
Q: Organic Chemistry Correct Separation Scheme * cannot be hand drawn *see attached image of…
A: Solvent extraction is the liquid-liquid extraction wherein the compounds are separated based on…
Q: For the molecule shown below, provide the names for all of the indicated functional groups: 1.…
A:
Q: In the reaction below, iodine and bromine react to give iodine monobromide. If the initial mixture…
A:
Q: 4. Fill in the complete set of reagents necessary for each of the following reactions. a. b. Но,
A:
Q: In acetic acid/sodium acetate buffer the solute particles were Na+, C₂H3O2, and HC₂H3O2. With which…
A: The given solute particles of the buffer are Na+, C2H3O2-, HC2H3O2Hydronium ions are strong donor of…
Q: From the following structures, select a reasonable resonance contributing structure for the molecule…
A:
Q: O: From the following structures, select a reasonable resonance contributing structure for the…
A: Resonance structures are sets of Lewis structures that describe the delocalization of electrons in a…
Q: Identify the type of nuclear decay that occurred in each of the following: (a) Ruthenium-102 was…
A: The symbols of alpha, beta, electron, and positron are respectively.
Q: 12. potassium iodate (0.6 g, 214 g/mol) was dissolved in 100 mL solution and 20 mL was mixed with…
A: 12) Mass of KIO3 dissolved in 100 mL solution= 0.6g Molar mass of KIO3 = 214 g / mol) Volume of KI…
Q: Choose correct reagent(s) for the conversion below al propanoyl chloride with (1) AlCl3 and (2) H₂0…
A: Friedel-craft alkylation: Benzene reacts with alkyl halide in the presence of Lewis acid to do…
Q: Consider the following choices when answering questions 86-89. b) c) P 808080 080808 808080 c.…
A: A question based on mixture of compounds. 5 pictures are given that are to be distinguished based on…
Q: For the reaction below, Kc = 4.60 × 10⁻⁶. Note Kc is sometimes called K. What is the equilibrium…
A:
Q: H C-Cl CH3 + NaOCH₂ SN2
A: The given reaction is an example of bimolecular nucleophilic substitution reaction. The product…
Q: I need help with two compounds that are some kind of isomers, the ones i picked are the optical…
A: tris-ethylenediaminecobalt(III) chlorideDeriving the structure:It has three ethylenediamine groups,…
Q: A chemist designs a galvanic cell that uses these two half-reactions: standard reduction…
A: Note: Since you have posted a question with multiple sub-parts, we will solve the first three…
Q: All resonance structures for HC(O)CHCHNH2
A: In resonance structure, the position of atomic nuclei remains the same and only the position of…
Q: r the following reaction, 37.3 grams of sulfuric acid are allowed to react with 42.5 grams of zinc…
A: Zn(OH)2 + H2SO4→ ZnSO4 + H2OZinc hydroxide…
Q: + 0 10
A: This question belong to Pericyclic reaction of organic compounds.Mechanism goes through [4+2]…
Q: Identify the class type of compound shown: H₂SO4 Strong Acid O Weak Base O Strong Base O Weak Acid
A: An acid is a substance that can donate H+ ions in aqueous solution.On the other hand, a base is a…
Q: Macmillan Learning Label the boxed functional groups in the antidepressant molecule, venlafaxine.…
A:
Q: Predict the approximate bond angles in each of the molecules. + CH3: SiCl:
A: Bond angle = ? -------------------------------------------
Q: 6. What factors affect the end-point sharpness in an acid/base titration?
A: Several factors can influence the end-point sharpness in an acid/base titration. The end-point is…
Q: 65. Which is the strongest oxidizing agent? Standard Reduction Potentials Eº Na Na + e Cd Cd2+ + 2e…
A: The correct answer for questions 65 and 66 is to be identified.
Q: What other substrates can be used to produce vinegar?
A: Vinegar is acetic acid solution, chemical formula of acetic acid is CH3COOH
Q: (a) Using Lewis symbols, make a sketch of the reaction between potassium and bromine atoms to give…
A: Lewis dot structures are the structures of atoms or molecules that have valence electrons of all…
Q: In the structure of 4-isopropyl-2,4,5-trimethylheptane, identify the primary (p), secondary (s), and…
A: Primary hydrogen is bonded to a carbon that is bonded to only one other carbon atom. Secondary…
Q: What will happen in the reactions below? Draw the mechanisms.... 1. H ☆ O H + Cl₂
A:
Q: The reaction A + B ↔ C is known to be in equilibrium at a certain temperature. When the…
A: Le-chatelier principle: According to this when a system at equilibrium is subjected to stress then…
Q: Predict the major product of the following reaction: CH₂CH₂CCI Pod AICI;
A:
Q: For the molecule shown below, provide the names for all of the indicated functional groups:…
A: Functional groups are specific arrangements of atoms within organic compounds that are responsible…
Q: The glass manufacturing industry produces wastewater as a byproduct of their processes, with the…
A: In the glass manufacturing industry, wastewater is generated as a byproduct of various processes,…
Q: The Pauli Exclusion Principle tells us that no two electrons in an atom can have the same four…
A:
Q: Macmillan Learning Assign formal charges to each atom in the two resonance forms of COC1₂. :ci -4…
A:
Q: A prochirality center is a precursor to a chirality center. Identify the prochirality centers (if…
A: A question based on introduction to organic chemistry. Two reactants are given…
Q: 2) Consider a molecule that is a ring of six carbons, each of which is bonded to a single hydrogen.…
A: Lewis structure - it is an canonical structure of the molecule proposed by Lewis which included the…
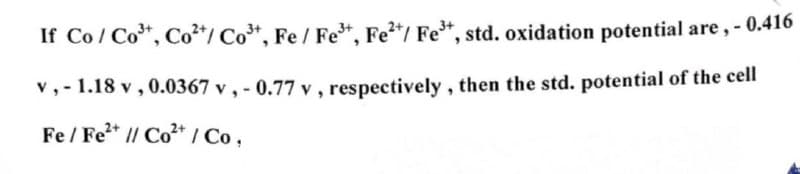
Step by step
Solved in 6 steps with 5 images
