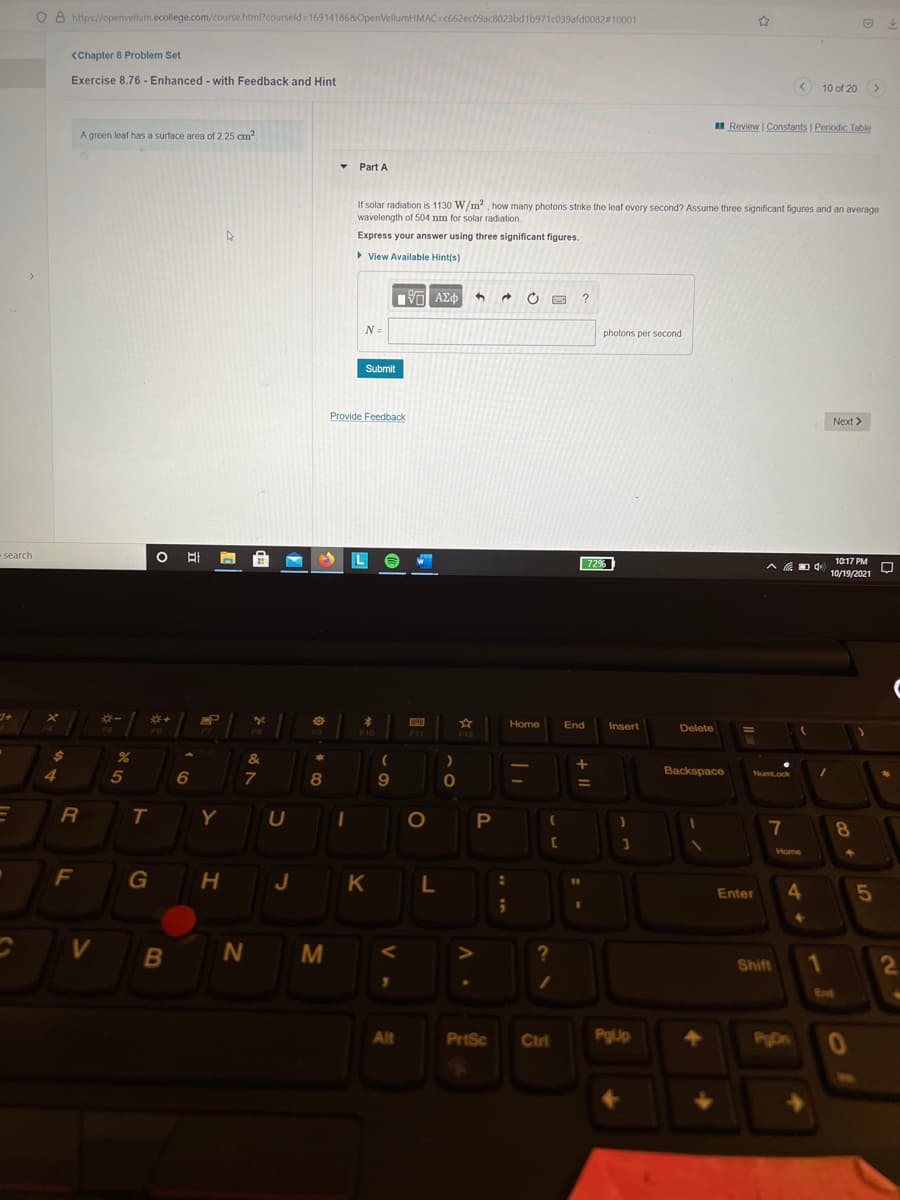
If solar radiation is 1130 W/m how many photons strike the leaf every second? Assume three significant figures and an average wavelength of 504 nm for solar radiation Express your answer using three significant figures.
If solar radiation is 1130 W/m how many photons strike the leaf every second? Assume three significant figures and an average wavelength of 504 nm for solar radiation Express your answer using three significant figures.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter19: The Representative Elements
Section: Chapter Questions
Problem 105CP
Related questions
Question
Transcribed Image Text:O 8 https://openvellum.ecollege.com/course.html?courseld=16914186&OpenVellumHMAC=c662ec09ac8023bd1b971c039afdo082#10001
<Chapter 8 Problem Set
Exercise 8.76 - Enhanced - with Feedback and Hint
10 of 20 >
II Review | Constants | Periodic Table
A green leaf has a surface area of 2.25 cm?
• Part A
If solar radiation is 1130 W/m2, how many photons strike the leaf every second? Assume three significant figures and an average
wavelength of 504 nm for solar radiation.
Express your answer using three significant figures.
> View Available Hint(s)
N =
photons per second
Submit
Provide Feedback
Next >
search
L
72%
10:17 PM
10/19/2021
P
Home
End
Insert
Delete
F10
F11
24
&
4
5
6
9
Backspace
Y
P
7
8.
Home
J
Enter
5
Shift
1
2
Ent
Alt
PrtSc
Ctri
PgUp
POn
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning