If the air temperature is the same as the temperature of your skin (about 30° C), your body cannot get rid of heat by transferring it to the air. In that case, it gets rid of the heat by evaporating water (sweat). During bicycling, typical 70 kg person's body produces energy at a rate of about 505 W due to metabolism, 76% of which is converted to heat. Part A How many kilograms of water must the person's body evaporate in an hour to get rid of this heat? The heat of vaporization of water at body temperature is 2.42 x 10° J/kg. Express your answer in kilograms. m = kg Submit Request Answer Part B The evaporated water must, of course, be replenished, or the person will dehydrate. How many 750 mL bottles of water must the bicyclist drink per hour to replenish the lost water? (Recall that the mass of a liter of wat is 1.0 kg.) Express your answer in bottles per hour. N = bottles per hour
If the air temperature is the same as the temperature of your skin (about 30° C), your body cannot get rid of heat by transferring it to the air. In that case, it gets rid of the heat by evaporating water (sweat). During bicycling, typical 70 kg person's body produces energy at a rate of about 505 W due to metabolism, 76% of which is converted to heat. Part A How many kilograms of water must the person's body evaporate in an hour to get rid of this heat? The heat of vaporization of water at body temperature is 2.42 x 10° J/kg. Express your answer in kilograms. m = kg Submit Request Answer Part B The evaporated water must, of course, be replenished, or the person will dehydrate. How many 750 mL bottles of water must the bicyclist drink per hour to replenish the lost water? (Recall that the mass of a liter of wat is 1.0 kg.) Express your answer in bottles per hour. N = bottles per hour
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter17: Energy In Thermal Processes: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 81P
Related questions
Question
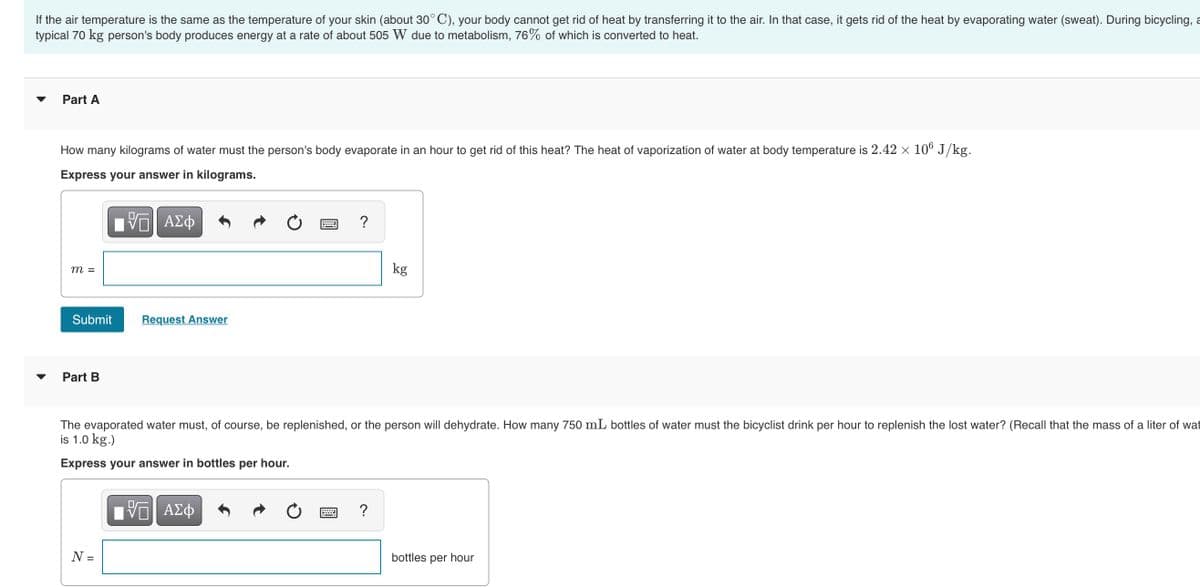
Transcribed Image Text:If the air temperature is the same as the temperature of your skin (about 30° C), your body cannot get rid of heat by transferring it to the air. In that case, it gets rid of the heat by evaporating water (sweat). During bicycling, a
typical 70 kg person's body produces energy at a rate of about 505 W due to metabolism, 76% of which is converted to heat.
Part A
How many kilograms of water must the person's body evaporate in an hour to get rid of this heat? The heat of vaporization of water at body temperature is 2.42 × 10° J/kg.
Express your answer in kilograms.
?
kg
m =
Submit
Request Answer
Part B
The evaporated water must, of course, be replenished, or the person will dehydrate. How many 750 mL bottles of water must the bicyclist drink per hour to replenish the lost water? (Recall that the mass of a liter of wat
is 1.0 kg.)
Express your answer in bottles per hour.
?
N =
bottles per hour
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning