Q: since the drug ketotifen has a methylpiperidine ring, does it mean it. has a chiral carbon and is an…
A: The objective of the question is to determine whether the drug Ketotifen, which contains a…
Q: Identify the missing reagent(s), reactant(s) or final product(s). Some transformations may require…
A: Given are organic reactions. Note: According to Bartleby Q&A guidelines we are supposed to…
Q: A student suggests that the molecule on the right can be made from a single molecule that doesn't…
A: The proper structure is drawn out in section below -Explanation:Here is the correct structure which…
Q: Draw and write only the main product of the reaction (C) Only typed solution
A: Organic reactions are reactions in which organic reactants react with each other to produce organic…
Q: Part 1 Select the carbons which contain hydrogen atoms that could be eliminated in an electrophilic…
A: In the given question we have to predict the hydrogen atoms that will be eliminated in an…
Q: 2) The product of the reaction shown is which of the following? Ο a. A ketone b. An alcohol c. An…
A: Final answer is given in explanation please see from there.Explanation:Approach to solving the…
Q: Draw all Newman projections of 2,2 bromopropane conformations in which the CH3 group and the H of…
A: The objective of the question is to find the Newman projections of dibromopropane conformations in…
Q: Ts Ts= ZO Dj O=S=O + 2 N=C= t-BuO K H NEC EtOH, CH2Cl2 4
A: Van Leusen reaction:This reaction involves conversion of ketones to nitriles with one additional…
Q: 40. An aqueous solution is 0.657 M in HCl. What is the molality of the solution if the density is…
A: To find the molality of the solution, we need to determine the number of moles of solute (HCl) per…
Q: onsider the following chemical equilibrium: 2H2(g) +0, (g) 2H₂O (1) ow write an equation below that…
A: Kp And Kc are the equilibrium constant of an ideal gaseous mixture. Kp is equilibrium constant used…
Q: || CH3-C-NH2 amine H J CH3-C-CH3 I OH CH₂ ။ 0=CH–CH CH CH₂ CH=C=0=C=CH, O D
A: A functional group is a group of atoms present in the molecule with different chemical properties,…
Q: This is the structure of 1-nonyne. Part: 0/2 Part 1 of 2 1-Nonyne reacts rapidly with NaH, forming a…
A: The given reaction is a reaction between an alkyne and sodium hydride (NaH). When alkyne reacts with…
Q: 8 6 5 How many distinct signals would appear in the (proton-decoupled) 13C NMR spectrum for the…
A: 13C-NMR spectra is used to find the carbon skeleton of the unknown compound.The chemically…
Q: Consider the ion below and assume that it is planar. Part: 0/2 Part 1 of 2 Determine the number of…
A: The cycloheptatrienyl anion, also known as the tropylium ion, has a planar, cyclic structure with…
Q: Which of the following Lewis structures will have resonance form H | H - с O-H H H-CEN - H C H ==0
A: Resonance structure : It is the group of more than one Lewis structure , that is representing a…
Q: hal Groups Predicting the reactants or products of esterification Predict the product of this…
A: When a carboxylic acid reacts with an alcohol in the presence of an acidic medium and heat formation…
Q: please help me with filling in the table below
A: Oxidative addition (i):Structure: [L-Pd(OTf)(X)]X-type ligands: 1L-type ligands: 3Oxidation state:…
Q: Give detailed Solution..show work..don't give Handwritten answer..don't use Ai for answering…
A: The objective of the question is to calculate the half-life of a zero-order reaction. The half-life…
Q: QUESTION 2c below. Your structure should clearly indicate any regiochemistry / stereochemist Draw…
A: A conjugated diene (a compound in which two C=C bonds are separated via a C-C bond) and a dienophile…
Q: The reaction A→B + C is zero order with respect to A. When [A]0 = 0.765 M, the reaction is 37.5 %…
A: We have to calculate the half-life of the given zero-order reaction.
Q: PLS HELP ASAP ON ALL ASKED QUESTIONS AND SHOW ALL WORK
A: (a) The enthalpy change of the reaction ΔH= - 12.1 kJ/mol(b) The potential energy diagram shows that…
Q: In a 1m3 sample of air, 3g of water that was heated so that all 3g evaporated. How many calories of…
A: 1m3 sample of air, 3g of water
Q: Hydrogen chloride and oxygen react to form water and chlorine, like this: -> 4HCl(g)+0₂(g)…
A: According to Le Chatelier principle if a system is in equilibrium is subjected to change of…
Q: 2) Provide an arow-pushing mechanism for the reaction shown below. HO OH [H2SO4] I
A: Diols react with aldehydes and ketones in the presence of an acid catalyst to yield acetals in a…
Q: PLS HELP ASAP ON ALL ASKED QUESTIONS AND SHOW ALL WORK
A: a. Step 1, i.e., 2B → Eb. Intermediates: E, K, EK and F c. Overall reaction: 2B+2A→D+C d. Catalyst:…
Q: Consider the following chemical equilibrium: H2(g) +CO₂ (g) H₂O(g) +CO (g) Now write an equation…
A: Equilibrium constant is defined as the ratio of concentration of products to the concentration of…
Q: When a 0.225-g sample of benzoic acid is combusted in a bomb calorimeter, the temperature rises…
A: ACCORDING TO GUIDELINES I HAVE TO DO ONLY FIRST QUESTION UNTIL MENTIONEDGIVEN -mass of benzoic acid…
Q: d. Oxalic acid (H2C2O4) is the compound that gives rhubarb its tart flavor, and it is also a toxin…
A: The question is based on the concept of molarity.Molarity is a method of expressing concentration of…
Q: PLS HELP ASAP ON ALL ASKED QUESTIONS AND SHOW ALL WORK
A: • The enthalpy change for combustion of acetic acid = 868.598 KJ/molExplanation:Step 1: Given,…
Q: Show work..don't give Handwritten answer
A: The objective of the question is to calculate the half-life of a zero-order reaction. The half-life…
Q: For the reaction below, Kc = 0.60 at 550 K. The reaction starts with a 0.050 M concentration of…
A: The chemical equilibrium constant, often denoted as Kc for concentration equilibrium, is a…
Q: 2. 13.9 g of ammonium nitrate is dissolved in a total volume of 225 mL in a coffee cup calorimeter.…
A: Given:Mass of ammonium nitrate dissolved = 13.9 gTotal volume = 225 mLInitial temperature of the…
Q: Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product…
A: The objective of the question is to predict the product formed in the following reaction.
Q: Gases in a Salt Marsh: You measure appreciable CH 4(g) andCO 2(g) partial pressures in the sediment…
A: The objective of the question is to calculate the redox potential (Eh) from the ratio of the…
Q: STARTING AMOUNT How many atoms of vanadium are in 1.28 grams of vanadium ?
A: We have to find how many atoms of vanadium are in 1.28 grams of vanadium.
Q: B 5 3 2 1 Hany peaks appear in the proton spin decoupled 13 C NMR spectrum of the compound bel CH
A: The objective of the question is to find the number of peaks that appear in the proton spin…
Q: The generic metal A forms an insoluble salt AB(s) and a complex AC, (aq). The equilibrium…
A:
Q: Calculate the pH of a 0.21 M solution of barium hydroxide. Express your answer in decimal notation…
A: Concentration of barium hydroxide solution = [Ba(OH)2] = 0.21 MpH of the solution = ?Note: Barium…
Q: Draw the product of this reaction and account for its formation, providing a curly arrow mechanism…
A: Reaction of benzene ring containing halogen group attached leads to the formation of benzyne as…
Q: 1. Predict the organic product(s) from the following reactions. Draw the curved arrows for the…
A: LDA (lithium Diisopropyl Amide) is a bulkier base that abstracts protons from less hindered side of…
Q: 4. Provide a mechanism for the following transformation: NaOH H2N. Н20, д
A:
Q: 2 pts Q3. Consider the following energy diagram: Potential Energy T.S. 1 I A T.S. 2 B Reaction…
A: The objective of the question is to understand the structure of the first transition state (T.S. 1)…
Q: CH3 CH3CH2OH
A: The objective of this question is to predict the product of the reaction between anisole and…
Q: Please don't provide handwritten solution ....
A: The objective of the question is to calculate the equilibrium constant in terms of concentration…
Q: aming and 1. Draw and name the following compounds ✓a. CH CHCHCHICH
A: Given are condensed structural formulas for organic compounds. Rules for determination of IUPAC…
Q: Draw the most likely conjugate base resulting from this acid- base reaction. Include all lone pairs.…
A: ->NaOEt is a base which can remove most acidic hydrogen from a molecule and form conjugate base.…
Q: QUESTION 2a between one equivalent of HBr with the compound shown below? What would be the major 1,2…
A: The objective of this question is to draw major 1,2 and 1,4 products formed by the reaction between…
Q: A student heats 84.17 mL of water to 95.27°C using a hot plate. The heated water is added to a…
A: During calorimetry, the heat lost by the hot water (qhot) will be the sum of heat energy gained by…
Q: Synthesize the following stereoisomers utilizing a strategy that makes the desired isomer a major…
A: In the given question we have to predict the reaction conditions for the formation of the desired…
Q: Determine whether each of the following compounds could be removed from an organic solvent by…
A: Solubility is the ability of a substance to interact with a solvent and form a solution. The ability…
Unlock instant AI solutions
Tap the button
to generate a solution
Click the button to generate
a solution
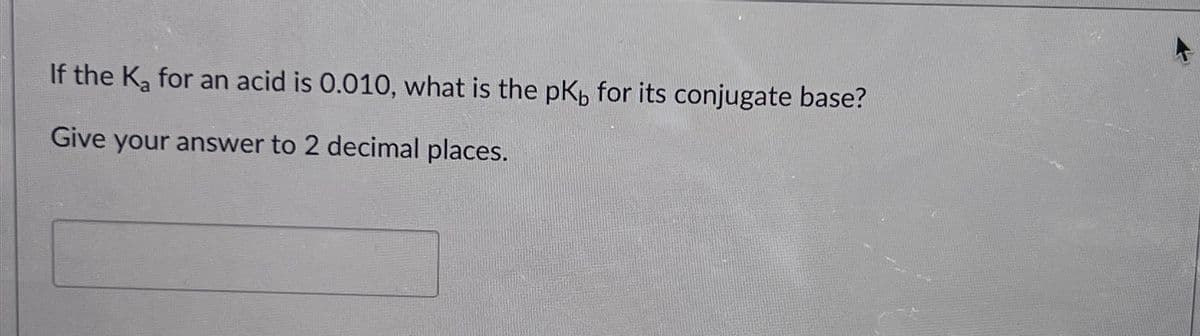
- Calculate the Kb value for the conjugate base of hydrosulfuric acid. The Ka for H2S is 9.6 x 10-8What is Kb for the conjugate base of CH₃COOH (Ka = 1.8 × 10⁻⁵)?Put the following bases in order from weakest to strongest and explain why. Cute specific pKa values of conjugate acids to answer this question.
- If the Ka of the conjugate acid is 5.06 × 10-5 , what is the pKb for the base?Which of the following two acids has the stronger conjugate base? Nitrous acid, Ka = 7.1 x 10–4 Phenol, Ka = 1.0 x 10–10 The strength of the conjugate base cannot be obtained from the information given. Nitrous acid, because it has a larger Ka value. Nitrous acid, because it has a smaller Ka value. Phenol, because it has a larger Ka value. Phenol, because it has a smaller Ka value.Use the Kb for the nitrite ion, NO2−, to calculate the Ka for its conjugate acid.
- Benozic acid (C6H5COOH) has a pKa of 4.20. a. What is the ratio of the acid to its conjugate base at a pH of 2.20?What is the conjugate base of H2S?Why is HCl so acidic? For example, why is H-Cl more acidic than H-S-CH3? Isn't the conjugate base's negative charge more stabilized with a sulfur and extra methyl group (by the induction effect)? While Cl is more electronegative than Sulfur, I would assume Cl being alone as its own ion would make it less stable.

