Q: Pbl, solid (Kps = 9.8 x 10-9) is placed in a beaker with water. After some time, the concentration…
A:
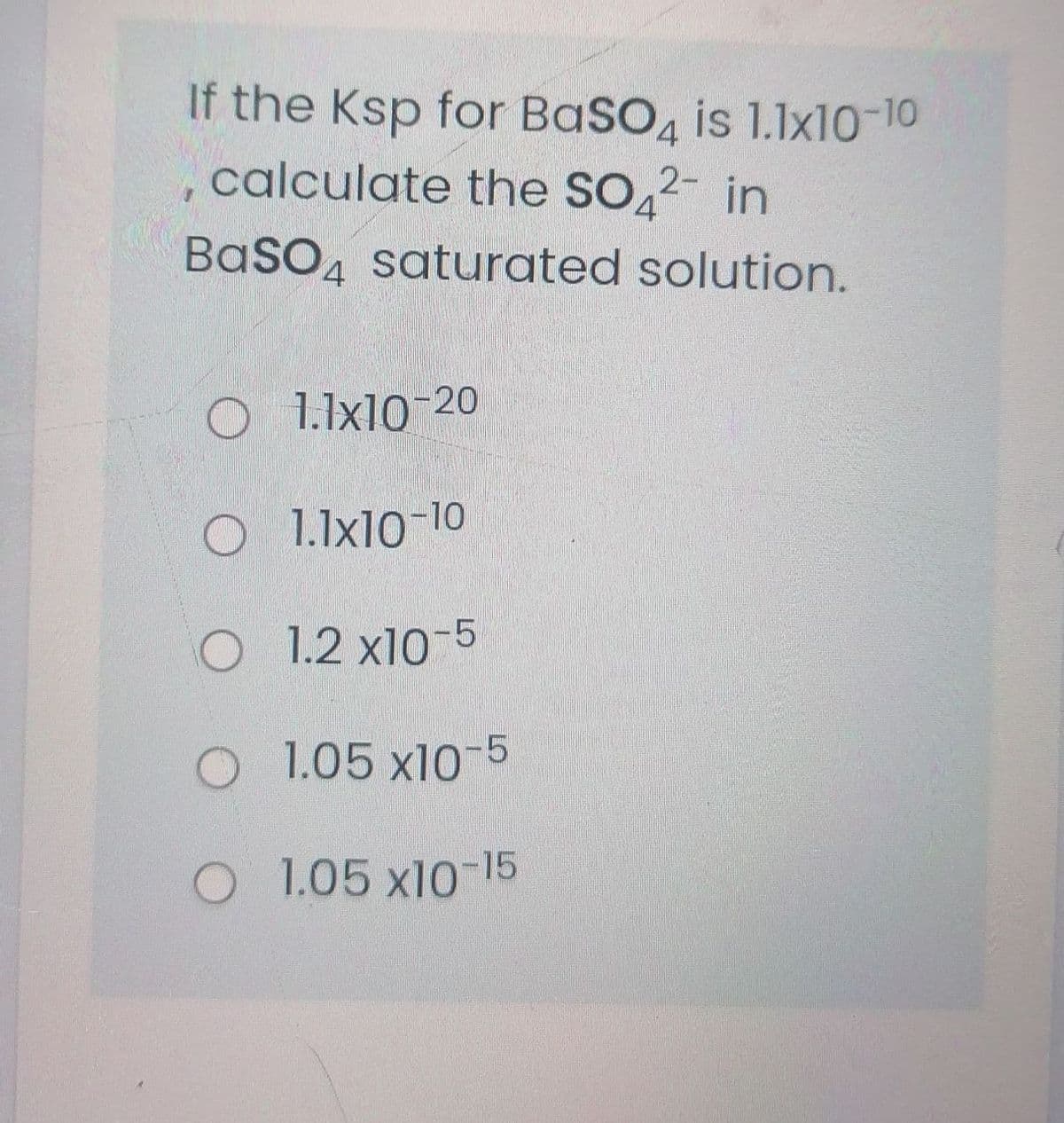
Q: At a particular temperature the value of [Ba2+] in a saturated solution of barium sulfate is…
A:
Q: The concentration of Pb* in a saturated solution of PBF2 is 2.10x10- mol/L at a certain temperature.…
A: PbF2 →Pb2+ + 2F- S 2S S is the solubility(or molarity) So , Ksp =…
Q: Calculate Ksp for calcium hydroxide if a saturated solution contains 0.20M of calcium hydroxide.
A: Ksp is tge solubility product of sparingly soluble compound. It can be calculated as the product of…
Q: Calculate the solubility at 25 °C of AgBr in pure water and in 0.45 M NaI, You'll probably find some…
A: Concept of solubility is used to find AgBr concentration.
Q: What is the pH at 25degrees Celsius of a saturated solution of silver hydroxide? (Ksp value for…
A:
Q: Calculate the molar solubility (in mol/L) of Ag2S(s) at 25 °C in a solution of 0.30 M Na2S(aq). Ksp…
A: The majority of ionic compounds are water-soluble. When a substance is soluble, it dissociates into…
Q: The molar solubility of PbBr₂ is 2.17 x 10-3 M at a certain temperature. Calculate Ksp for PbBr2.…
A: The given multiple choice do not contain the correct answer.
Q: The molar solubility of insoluble salt with the generic formula, MX, is 9.1 × 10−9 M. Calculate Ksp…
A: Ksp = [M+ ][X-]
Q: Calculate the solubility (in g/L) of CaSO,(s) in 0.400 M Na, SO,(aq) at 25°C. The Ksp of CaSO, is…
A: CaSO4 is a sparingly soluble salt and it ionises as Ca+2 and SO42- ions Now , Ksp is called…
Q: Assuming that the solubility of Ca3(PO4)2(s) is 1.6×10-7 mol/L at 25°C, calculate the Ksp for this…
A:
Q: The tartaric acid, H2C4H406 (150.087 g/mol ) concentration in 100.0 mL wine sample was determined…
A: Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits ,like grapes…
Q: Calculate the [Ag"] and [CrO42], and the solubility of Ag CrO4 in a solution prepared by adding an…
A: Given: Moles of Na2CrO4 = 0.10 mol Volume of Na2CrO4 = 1 L Ksp of Ag2CrO4 = 1.9×10-12
Q: 25 mL of a saturated solution of K2Cr2O7 was cooled from 90oC to 50oC, how many moles solid will…
A: From the plot it is noticed that Solubility of K2Cr2O7 at 90°C is = 70 g/100g of water Solubility…
Q: 14. The Kp of PbSO, in water at 25°C is 2.13 X 108. It's molar solubility is ...
A: Given,
Q: Calculate the mas of AgCl need to contain 7.5g Ag
A: Given, Mass of Ag = 7.5g. The reaction of Ag to give AgCl is
Q: (c) (a) Pure Pure Composition reactants products For the three curves, what is the common feature of…
A: The given graph is represented as follows:
Q: Titarant in Mohr's method is AgNO; solution; KSCN solution; Hg2(NO;), solution; NaCl solution
A:
Q: In a gravimetric determination of a soluble sulfate the data gathered is presented (see attachment).…
A: To calculate the mean of trials for sample 1 and 2 for empty crucibles and crucibles with…
Q: What happens when too much NaSCN is prepared in solution of mixtures of standard solutions of…
A: Fe3+ (from Fe(NO3)3 and SCN- ions (from NaSCN) react to form a complex.
Q: Assuming that the solubility of PbCl2(s) is 1.6×10-2 mol/L at 25°C, calculate the Ksp for this salt.…
A: Solubility=1.6x10-2 mollitrePbCL2s→Pb+2aq+2Cl-aq S 2S2Ksp=4s3
Q: Calculate the pH of a titration mixture when 25.36 mL of 0.081 M NaOH has been added to a 50.00 mL…
A: In this question we have to tell the PH of the mixture of the given solution. This solution create a…
Q: How much (in g) of Calcium hydroxide is required to increase the pH of a 100mL solution containing…
A: Buffer solution is a solution of acid and it's conjugate base or salt. This solution has constant pH…
Q: Given the equation Ag*(aq) + 2 NH, (aq) – [Ag(NH,),]*(aq) Kf = 2.00 × 107 determine the…
A: Given: Mass of AgCl to be dissolved = 529 mg = 0.529 g. Volume of solution = 100.0 mL = 0.100 L…
Q: Determine the pOH of a 0.427 M C5H5N basic solution at 25°C. The Kp of C5H5N is 1.7 x 10-9.
A: We have given that Determine the pOH of 0.427M C5H5N basic solution at 25°C the Kb of C5H5N is 1.7…
Q: Calculate the solubility (in g/L) of CaSO̟(s) in 0.500 M Na, SO̟(aq) at 25°C. The Ksp of CaSO, is…
A: The solubility of a salt decreases in the presence of common ion. The anion (SO42-) is common in…
Q: Which of the following anions will separate barium ions from calcium ions most effectively by…
A: Precipitation reactions are those reaction in which a highly insoluble salt is formed as the…
Q: 10p in recent years, technology has developed considerably in analytical devices as in many areas…
A:
Q: A saturated solution of lead(II) sulfate can be prepared by diluting 0.0183 g of PbSO4 (MM= 303.26…
A: We have to find the Ksp of PbSO4
Q: The concentration of Sr2* in a saturated solution of SrCrO4 is 6.29x10-3 mol/L at a certain…
A:
Q: Given the equation Ag*(aq) + 2 NH, (aq) [Ag(NH,),J* (aq) K¢ = 2.00 x 107 determine the concentration…
A: Answer: AgCl will react with excess NH3 and dissolved. The equilibrium constant of the reaction is…
Q: What is the solubility constant of magnesium hydroxide if 0.019g of magnesium chloride is dissolved…
A: Given that: pH = 10 Mass of MgCl2 = 0.019g M.w = 95.21g/mol Moles of MgCl2 = 0.019g÷95.21g/mol =…
Q: How many moles of AgCl can dissolve in a solution that contains 0.001 mol/L NaCl, given the Ksp for…
A: The answer explained below.
Q: 2- The concentration of SO, in a saturated solution of Hg2SO4 is 5.54x10-3 mol/L at a certain…
A: Given- Concentration of SO42- = 5.54 × 10-3 Mole/L Ksp of the compound=?
Q: When silver chromate, Ag2CrO4, dissolves in otherwise pure water, what is the relation between [Ag+]…
A: Solubility product ( Ksp ) :- In a saturated solution of sparingly soluble salt there will be an…
Q: 14. The Kp of PbSO, in water at 25°C is 1.23 X 107. It's molar solubility is ....
A: Please note- As per our company guidelines we are supposed to answer only one question. Kindly…
Q: At a particular temperature, the solubility of In₂(SO₄)₃ in water is 0.0077 M. What is the value of…
A: Ksp is the kind of equilibrium constant when a solid substance dissolves into an aqueous solution.…
Q: The concentration of an aqueous solution of CaSO4 (Ksp = 7.1 × 10–5) is 8.43 × 10–3 M. Qsp = ___ and…
A: Ksp stand for solubility product. Qsp stands for Solubility Product Quotient and is used to…
Q: The solubility of Mg(OH), in water at 25 °C is measured to be 0.0096 Use this information to…
A: Mg(OH)2 ionizes as follows Mg(OH)2 -----------> Mg2+ + 2OH- Initial. S.…
Q: In which of the following solutions would the solubility of PbCl2 be the lowest?
A: c) NaCl solution
Q: prepare standards by conducting a serial dilution of 2mg/ml BSA down to 0.0625mg/ml BSA. How many…
A: Serial dilutions involves simultaneous dilution steps that helps in obtaining highly diluted…
Q: Calculate for the molar solubility of stannous sulfide Ksp = 1.0 x 10-26
A:
Q: When all of the following are mixed together in a beaker, what is the molar concentration of the…
A: The question is based on the concept of solutions. we have to calculate concentration of the…
Q: Calculate the solubility (in g/L) of CaSO, (s) in 0.300 M Na, SO,(aq) at 25°C. The Ksp of CaSO, is…
A:
Q: 1. The solubility of copper(I) chloride is 0.0391 g/L. Calculate the Ksp for copper(I) chloride
A:
Q: At a particular temperature, the solubility of In₂(SO₄)₃ in water is 0.0060 M. What is the value of…
A: Ksp is used to denote the solubility product which may be calculated by the product of concentration…
Q: What of the following is the expression for the solubility product of Ba3(AsO4)2?
A: Given-> Ba3(AsO4)2
Q: Calculate the molar solubility of BaCrO4 Barium Chromate at: • Water • 0.0500 mol.L-1 solution of…
A: Molar solubility from BaCrO4 can be calculated if we know the Ksp value of BaCrO4 which is…
Q: A saturated solution of AB2 contains 8.60 x 10.4 moles AB2 per liter. Determine Ksp.. 2.54 x 10-⁹…
A:
Step by step
Solved in 2 steps

- Consider a saturated solution of barium iodate in 0.025 M BaCl2. For the salt: Ba(IO3)2(s) ⇌ Ba2+ + 2IO3- Ksp = 1.57 × 10-9 at 25 deg C What is the activity coefficient of Ba2+?. 0.439 0.364 0.276 0.405Calculate Ksp value for Ce(IO3)4, solubility = 1.8x10-4 mol/LThe solubility of Fe(OH)3 is measured and found to be 2.30×10-8 g/L. Use this information to calculate a Ksp value for iron(III) hydroxide.
- Benzophenone has a freezing point of 49.00oC. A 0.450 molal solution of urea in this solvent has a freezing point of 44.59oC. Find the freezing point depression constant for the solvent.Is Ksp for Mg3(PO4)2 is 5.2 x 10^-24 at 25 degree Celsius. What is the molar solubility of Mg3(PO4)2 in 0.25 NaPO4? -> 2.1 x 10^-10M -> 1.5 x 10^-8 M -> 1.5x10^-3M -> 4.1x 10^-1M -> 2.0 MAn equimolar solution of ammonium sulfate, sodium chloride, and magnesium nitrate has an ionic strength of 0.15M. What is molar concentration of the solution wiht respect to each solute? a. 0.010 b. 0.015 c. 0.020 d. 0.025
- Calculate the solubility of Ba(IO3)2 in pure water, Ksp=1.5x10^-9. Then calculate the solubility of the same Ba(IO3)2 in a 0.0025M Al(NO3)3 solution. Assume there is no other interatcion between any of the ionic componets of the 2 molecules. (do an activity coeffiecient problem using the neatest u value to determine f from the table)Calculate the solubility at 25 Celsius of CuBr in pure water and in a .0040 M CoBr2 solution. ksp of CuBr is 6.27x 10^-9 aditional information in the picture.The solubility of aqueous PbCr2O7 at 25 oC is 1.55 x 10–2 g/L at 25.0 oC. Calculate the Ksp of PbCr2O7 at this temperature. MM PbCr2O7 = 423.188 g/mol
- Calculate the solubility at 25°C of CuBr in pure water and in a 0.0060M CoBr2 solution. You'll find Ksp data in the ALEKS Data tab. Round both of your answers to 2 significant digits.Consider a saturated solution of barium iodate in 0.025 M BaCl2. For the salt: Ba(2IO3)^2(s) = Ba2+ + 2IO3^- Ksp= 1.57 × 10-9 at 25 deg C What is the activity coefficient of Ba2+? 0.276 0.439 0.405 0.364The solubility of Ni(OH)2 in a pH = 7.64 solution is___. Ksp Ni(OH)2 = 1.6 × 10-16. Enter the result in scientific notation to 1 decimal. e.g. enter 5.6x10-5 as 5.6E-5.

