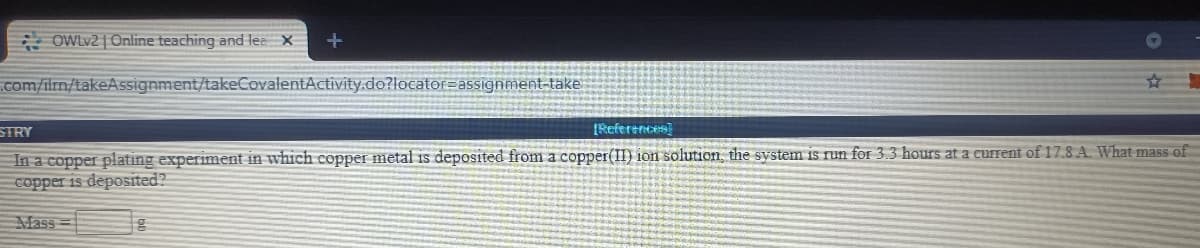
In a copper plating experiment in which copper metal is deposited from a copper(II) ion solution the system is run for 3.3 hours at a current of 17.8 A. What mass of copper is deposited? Mass
In a copper plating experiment in which copper metal is deposited from a copper(II) ion solution the system is run for 3.3 hours at a current of 17.8 A. What mass of copper is deposited? Mass
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter19: Principles Of Chemical Reactivity: Electron Transfer Reactions
Section: Chapter Questions
Problem 48PS: In the electrolysis of a solution containing Ag+(aq), metallic Ag(s) deposits on the cathode. Using...
Related questions
Question
Transcribed Image Text:* OWLV2 | Online teaching and lea X
.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take
STRY
IReferences
In a copper plating experiment in which copper metal is deposited from a copper(II) ion solution, the system is run for 3.3 hours at a current of 17.8 A. What mass of
copper is deposited?
Mass
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning