In a laboratory experiment, the density of a concentrated sugar solution was determined by measuring the volume of the solution and corresponding mass at room temperature. Five students each made a set of measurements (totally 15 measurements) using a different balance and used the results to calculate density (g/mL). Their results are summarized
In a laboratory experiment, the density of a concentrated sugar solution was determined by measuring the volume of the solution and corresponding mass at room temperature. Five students each made a set of measurements (totally 15 measurements) using a different balance and used the results to calculate density (g/mL). Their results are summarized
Algebra: Structure And Method, Book 1
(REV)00th Edition
ISBN:9780395977224
Author:Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Chapter12: Quadratic Functions
Section12.6: Solving Problems Involving Quadratic Equations
Problem 1.3E
Related questions
Topic Video
Question
can you please answer question b
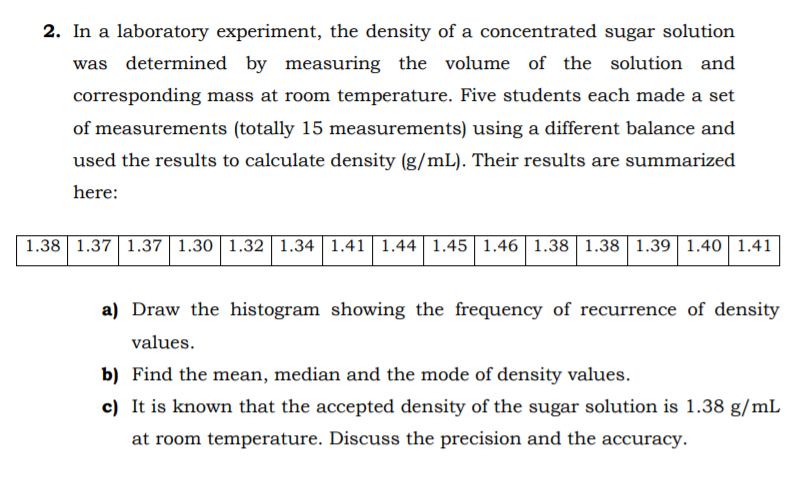
Transcribed Image Text:2. In a laboratory experiment, the density of a concentrated sugar solution
was determined by measuring the volume of the solution and
corresponding mass at room temperature. Five students each made a set
of measurements (totally 15 measurements) using a different balance and
used the results to calculate density (g/mL). Their results are summarized
here:
1.38 1.37 1.37 1.30 | 1.32 1.34 | 1.41| 1.44| 1.45| 1.46| 1.38 | 1.38 | 1.39 1.40 1.41
a) Draw the histogram showing the frequency of recurrence of density
values.
b) Find the mean, median and the mode of density values.
c) It is known that the accepted density of the sugar solution is 1.38 g/mL
at room temperature. Discuss the precision and the accuracy.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, statistics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Algebra: Structure And Method, Book 1
Algebra
ISBN:
9780395977224
Author:
Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:
McDougal Littell

Algebra: Structure And Method, Book 1
Algebra
ISBN:
9780395977224
Author:
Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:
McDougal Littell