In a terrible accident, a solution consisting of 2.37 kg of nitric acid, HNO3, was spilled on the lab floor. Very quickly. you decide to throw 2.00 kg of sodium carbonate, Na₂CO3, on the spilled acid. You then run out of the building due to the significant quantities of carbon dioxide that would form due to the reaction. As you stand outside, you wonder, "Did I add enough sodium carbonate to neutralize the acid?". a) Did you add enough sodium carbonate to neutralize the acid? O Yes O No b) In the event that you did not add enough sodium carbonate, how much extra would be needed to completely neutralize the acid? If you have added enough, please enter a value of 0 (zero). Needed o c) In the event that you did add enough sodium carbonate, how much extra did you add? If you didn't add any extra, please enter a value of 0 (zero). Excess 0
In a terrible accident, a solution consisting of 2.37 kg of nitric acid, HNO3, was spilled on the lab floor. Very quickly. you decide to throw 2.00 kg of sodium carbonate, Na₂CO3, on the spilled acid. You then run out of the building due to the significant quantities of carbon dioxide that would form due to the reaction. As you stand outside, you wonder, "Did I add enough sodium carbonate to neutralize the acid?". a) Did you add enough sodium carbonate to neutralize the acid? O Yes O No b) In the event that you did not add enough sodium carbonate, how much extra would be needed to completely neutralize the acid? If you have added enough, please enter a value of 0 (zero). Needed o c) In the event that you did add enough sodium carbonate, how much extra did you add? If you didn't add any extra, please enter a value of 0 (zero). Excess 0
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter6: Chemical Reactions: An Introduction
Section: Chapter Questions
Problem 20QAP: Many over-the-counter antacid tablets are now formulated using calcium carbonate as the active...
Related questions
Question
Chemistry
part c is incorrect the answer is not 0.
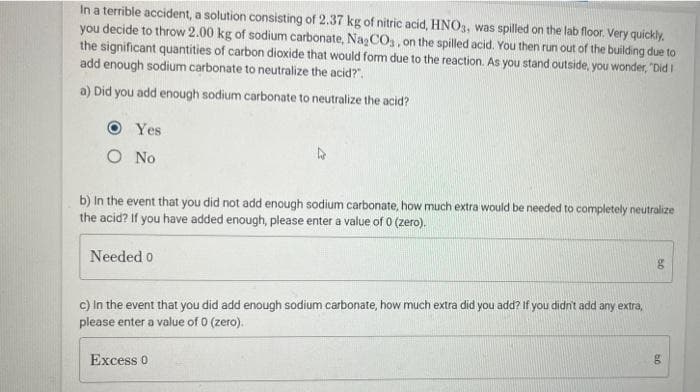
Transcribed Image Text:In a terrible accident, a solution consisting of 2.37 kg of nitric acid, HNO3, was spilled on the lab floor. Very quickly,
you decide to throw 2.00 kg of sodium carbonate, Na₂CO3, on the spilled acid. You then run out of the building due to
the significant quantities of carbon dioxide that would form due to the reaction. As you stand outside, you wonder, "Did I
add enough sodium carbonate to neutralize the acid?".
a) Did you add enough sodium carbonate to neutralize the acid?
O
Yes
O No
b) In the event that you did not add enough sodium carbonate, how much extra would be needed to completely neutralize
the acid? If you have added enough, please enter a value of 0 (zero).
Needed o
c) In the event that you did add enough sodium carbonate, how much extra did you add? If you didn't add any extra,
please enter a value of 0 (zero).
Excess 0
50
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning