In addition to the NMR spectrum below, this compound, with formula C5H,0O2, has bands at 3450 cm 1 (wide) and 1713 cm 1 (strong) in the infrared spectrum. Draw your structure note: point out the structures on the chart for better understanding
In addition to the NMR spectrum below, this compound, with formula C5H,0O2, has bands at 3450 cm 1 (wide) and 1713 cm 1 (strong) in the infrared spectrum. Draw your structure note: point out the structures on the chart for better understanding
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.7QAP
Related questions
Question
26. In addition to the NMR spectrum below, this compound, with formula C5H,0O2, has bands at 3450 cm 1 (wide) and 1713 cm 1 (strong) in the infrared spectrum. Draw your structure
note: point out the structures on the chart for better understanding
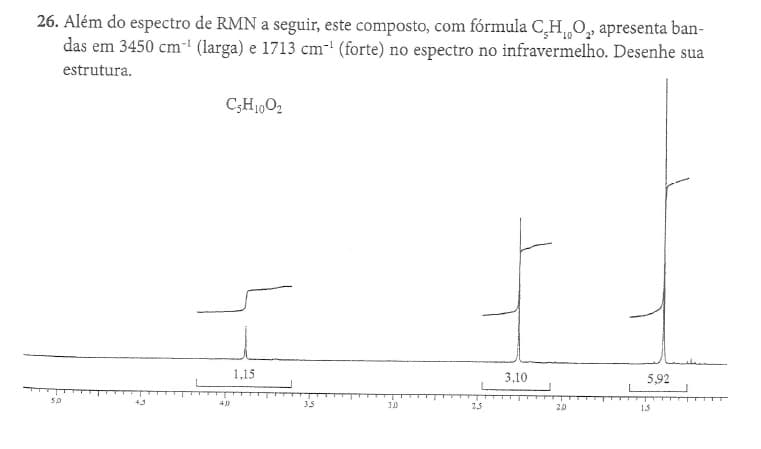
Transcribed Image Text:26. Além do espectro de RMN a seguir, este composto, com fórmula CH 0, apresenta ban-
das em 3450 cm- (larga) e 1713 cm-' (forte) no espectro no infravermelho. Desenhe sua
estrutura.
1,15
3,10
5,92
35
2,5
20
15
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
