In an experiment, 27.97 mL of 0.131 M LIOH is required to complete neutralize a 13.18 mL of phosphoric acid: H3PO4 (aq) + 3LIOH (aq) --> Li3PO4 (aq) + 3H2O (1) What is the molarity of the phosphoric acid? Answer with correct units and significantigures.
In an experiment, 27.97 mL of 0.131 M LIOH is required to complete neutralize a 13.18 mL of phosphoric acid: H3PO4 (aq) + 3LIOH (aq) --> Li3PO4 (aq) + 3H2O (1) What is the molarity of the phosphoric acid? Answer with correct units and significantigures.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 100E: Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory as a...
Related questions
Question
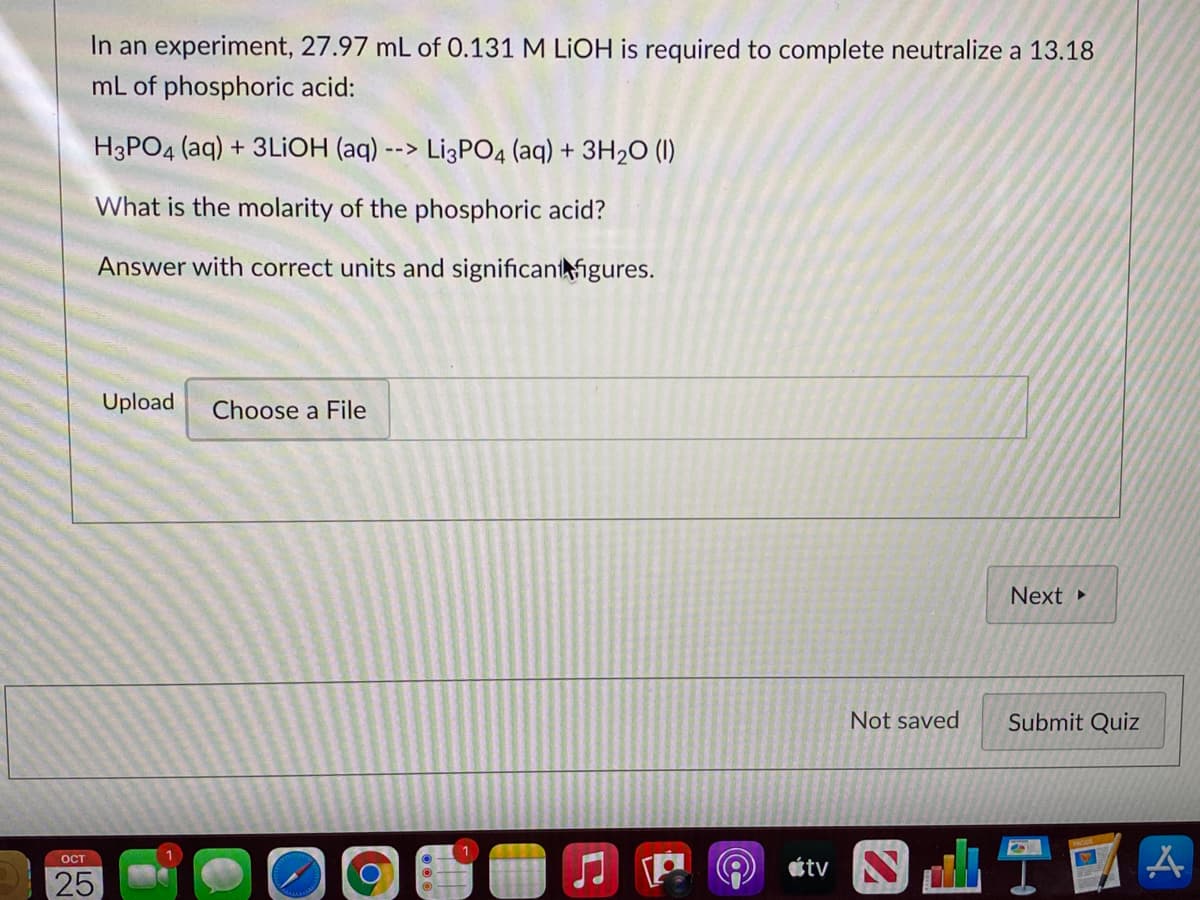
Transcribed Image Text:In an experiment, 27.97 mL of 0.131 M LIOH is required to complete neutralize a 13.18
mL of phosphoric acid:
H3PO4 (aq) + 3LIOH (aq) --> Li3PO4 (aq) + 3H2O (1I)
What is the molarity of the phosphoric acid?
Answer with correct units and significantigures.
Upload
Choose a File
Next
Not saved
Submit Quiz
étv N
ост
25
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT