In an experiment with an Ostwald Viscometer, the times of flow of water and toluene are 45 s and 32.9 s respectively at 30°C. The density of water is 1.002 g/cc. Calculate the density of the toluene in g/ml. (uwater = 0.8916 CP, utoluene = 0.5239 cP) Use 4 decimal places. * 0.8080 0.8053 0.8853 0.8882
In an experiment with an Ostwald Viscometer, the times of flow of water and toluene are 45 s and 32.9 s respectively at 30°C. The density of water is 1.002 g/cc. Calculate the density of the toluene in g/ml. (uwater = 0.8916 CP, utoluene = 0.5239 cP) Use 4 decimal places. * 0.8080 0.8053 0.8853 0.8882
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.13QAP
Related questions
Question
100%
kindly help me with this simple question from which is the correct answer
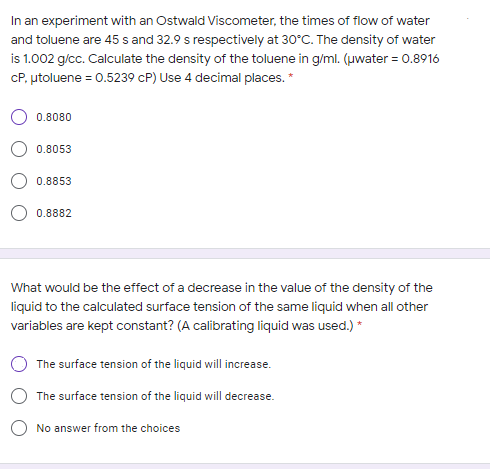
Transcribed Image Text:In an experiment with an Ostwald Viscometer, the times of flow of water
and toluene are 45 s and 32.9 s respectively at 30°C. The density of water
is 1.002 g/cc. Calculate the density of the toluene in g/ml. (uwater = 0.8916
CP, utoluene = 0.5239 cP) Use 4 decimal places. *
0.8080
0.8053
0.8853
0.8882
What would be the effect of a decrease in the value of the density of the
liquid to the calculated surface tension of the same liquid when all other
variables are kept constant? (A calibrating liquid was used.) *
The surface tension of the liquid will increase.
The surface tension of the liquid will decrease.
O No answer from the choices
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole



Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning